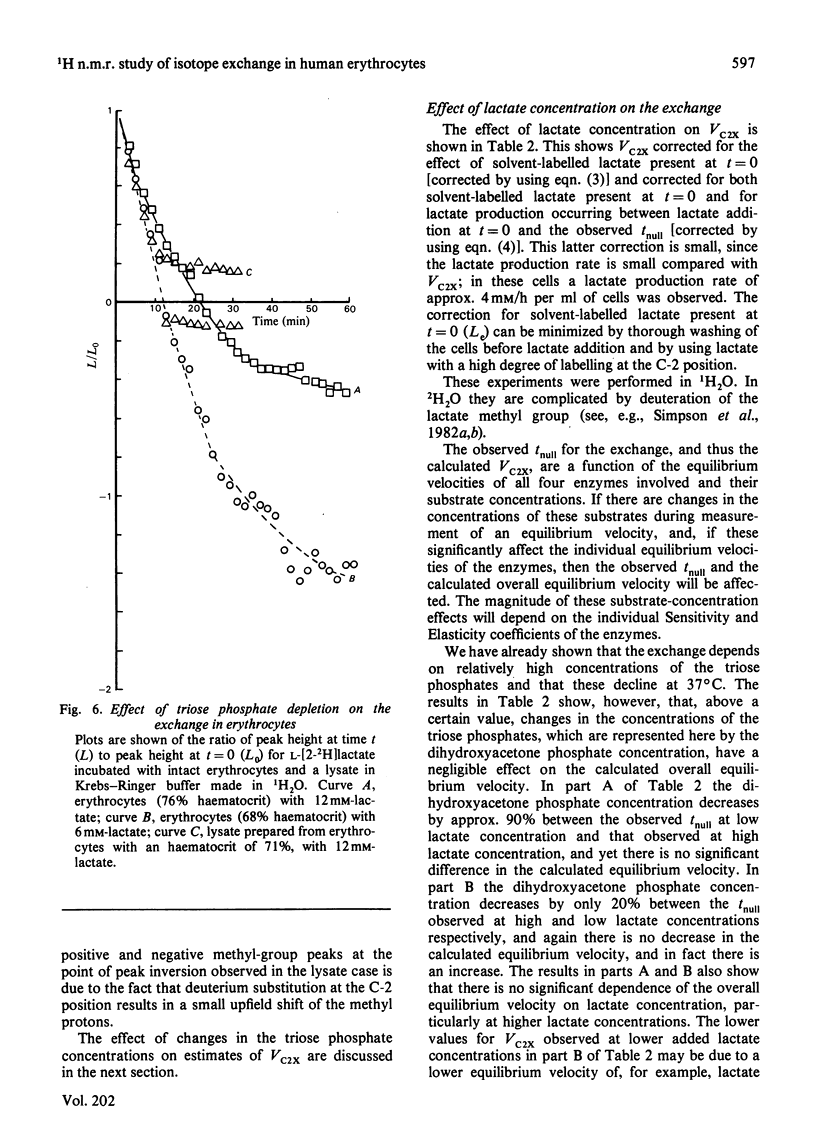
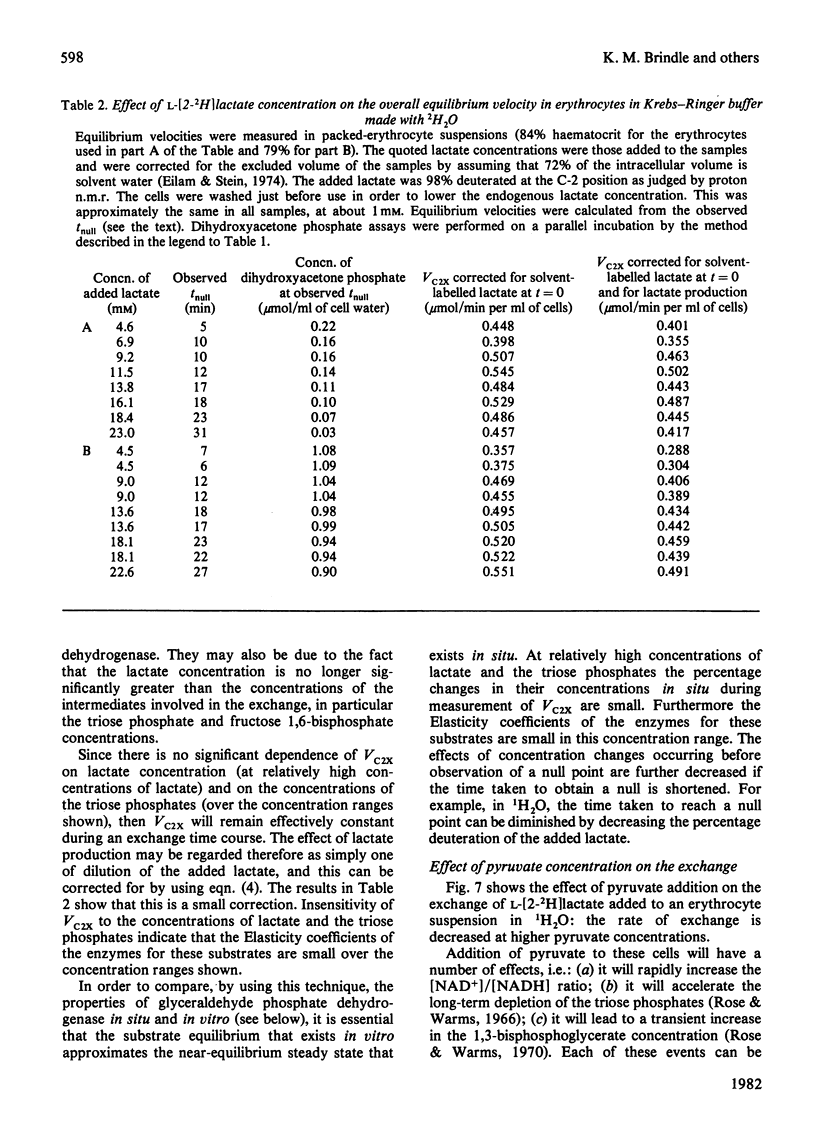
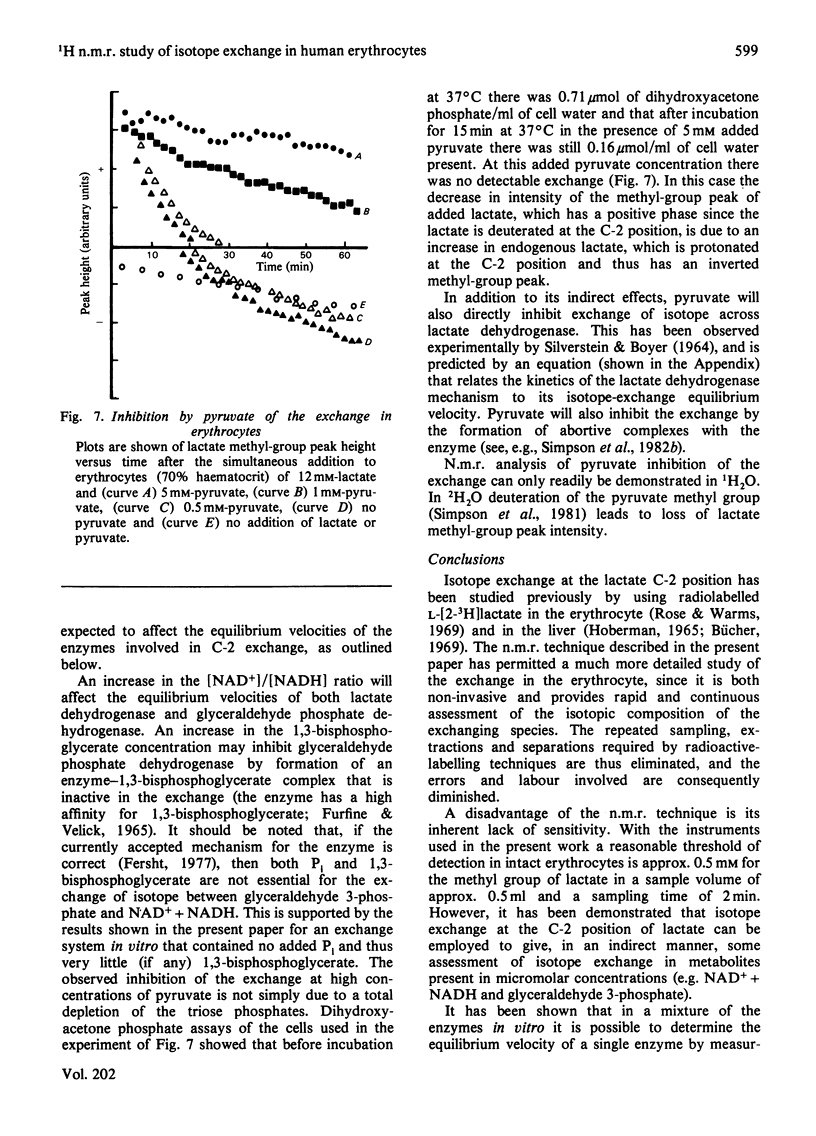
Abstract
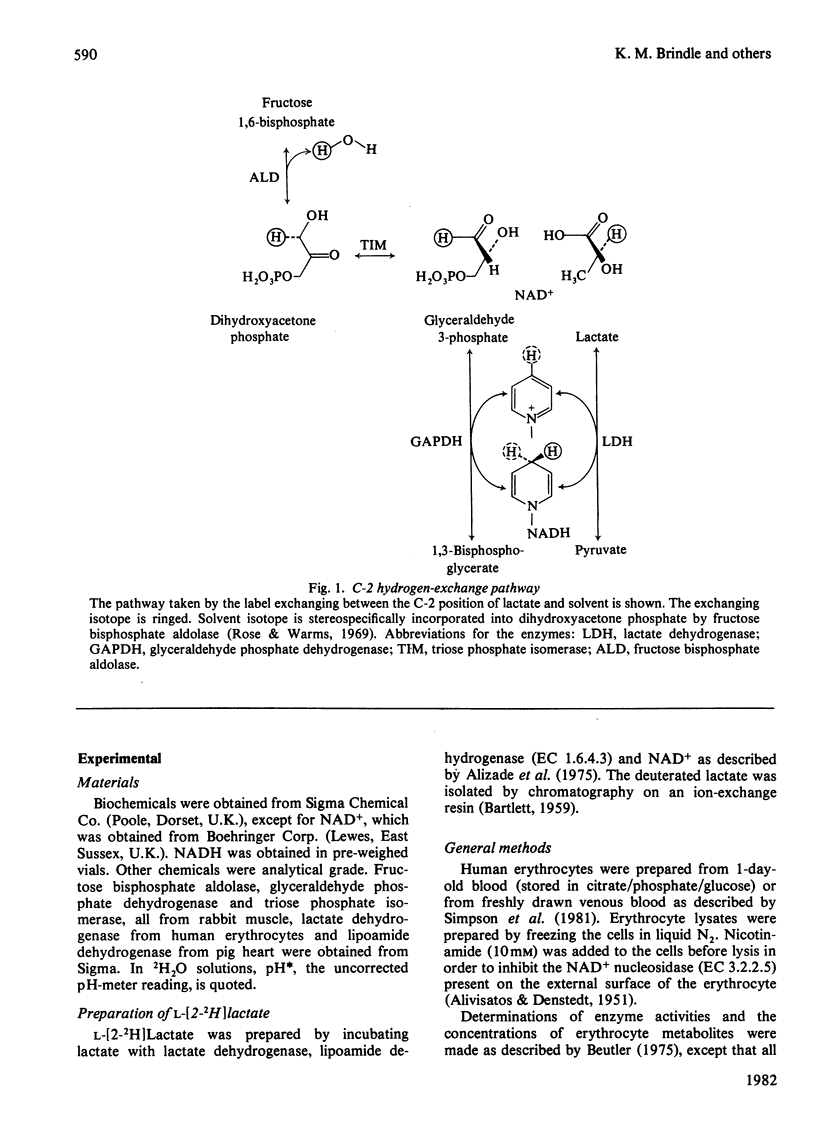
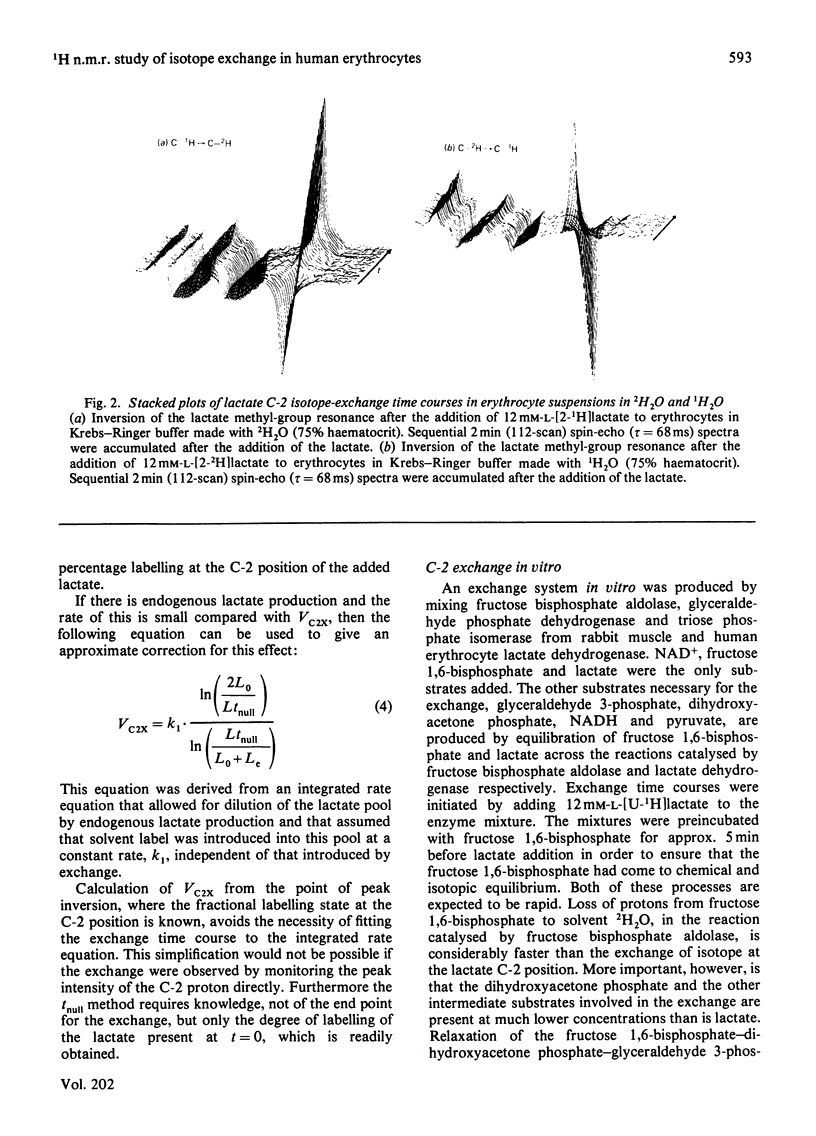
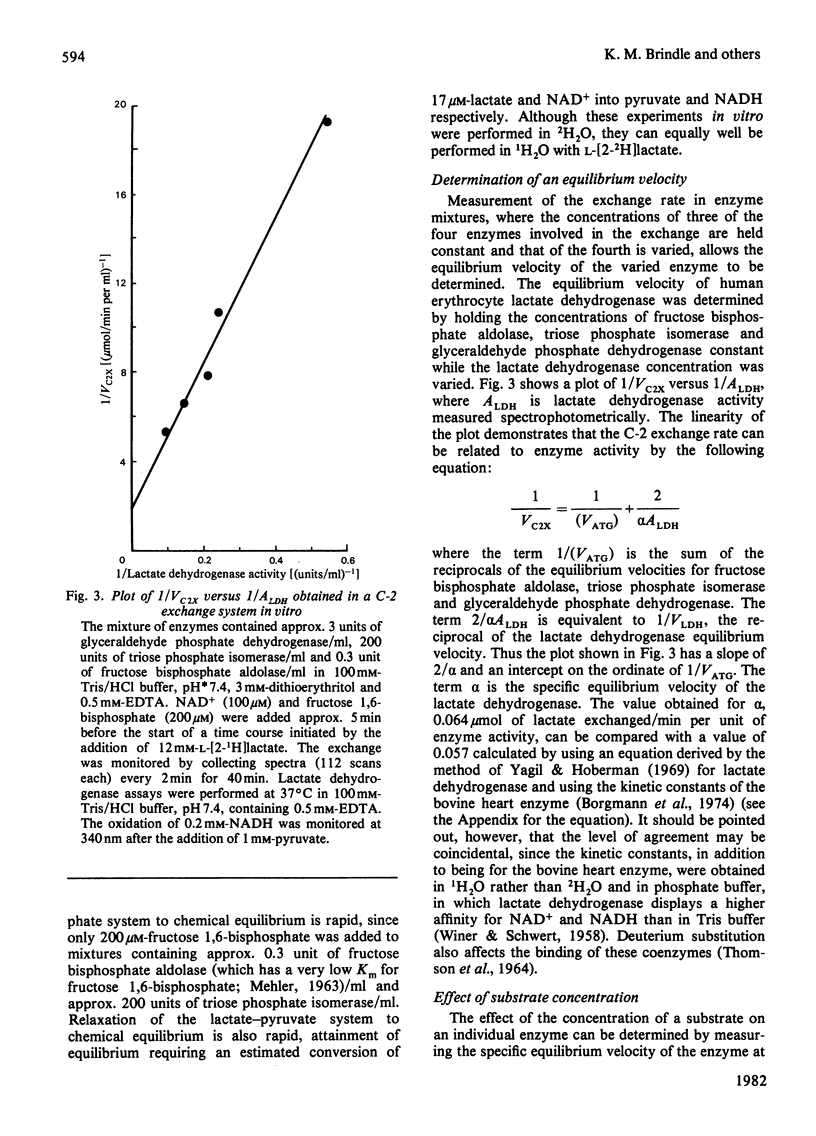
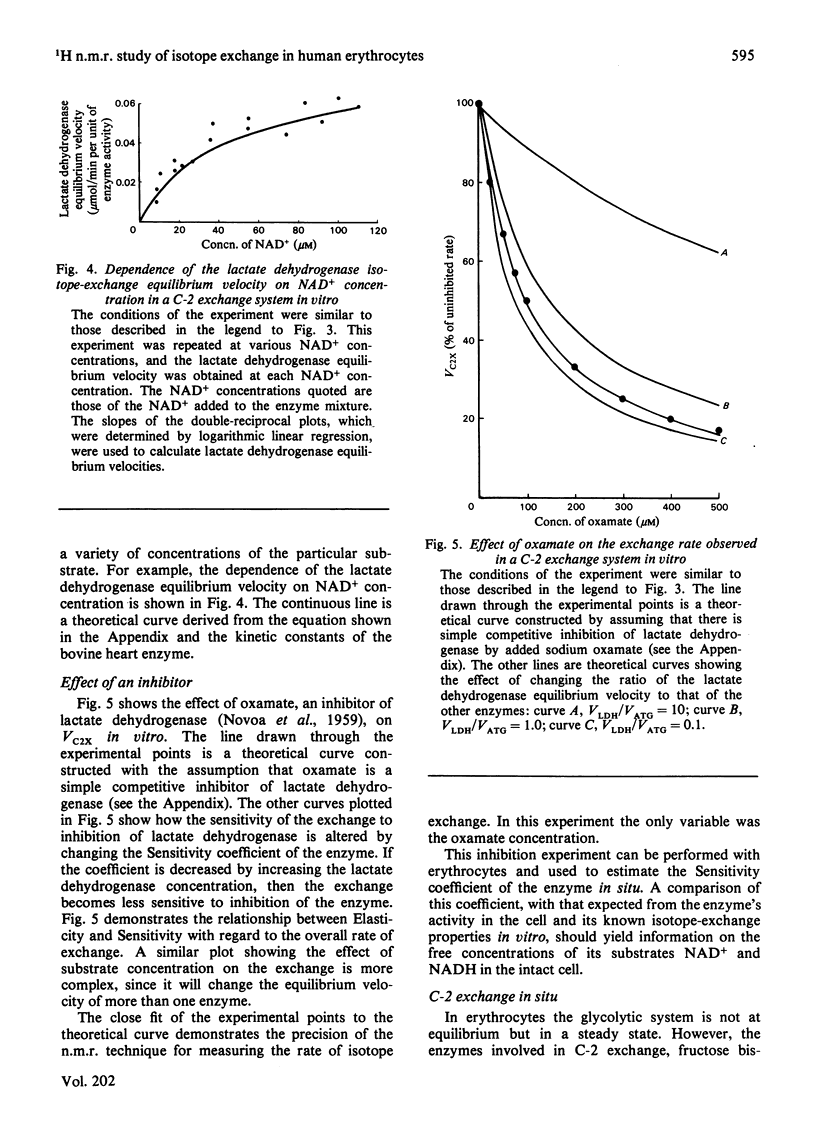
The exchange of hydrogen and deuterium atoms between the C-2 position of lactate and solvent was monitored in suspensions of human erythrocytes by using a non-invasive spin-echo p.m.r. method that permits continuous assessment of the rate and the extent of exchange. Exchange rates were measured in cells suspended in buffers made in 2H2O and 1H2O after the addition of L-[2-1H]lactate and L-[2-2H]lactate respectively. The rate of exchange is dependent on the activities of four glycolytic enzymes (fructose bisphosphate aldolase, triose phosphate isomerase, glyceraldehyde phosphate dehydrogenase and lactate dehydrogenase) and on the concentrations of their substrates. The dependence of the exchange on the following substrates was studied: (1) lactate, (2) the triose phosphates and fructose 1,6-bisphosphate and (3) pyruvate. Observation of the exchange in vitro, in a system produced by mixing the isolated enzymes, permits determination of the individual isotope-exchange equilibrium velocities of the enzymes. The dependence of the equilibrium velocity of human erythrocyte lactate dehydrogenase on NAD+ + NADH concentration was measured. Possible applications of these methods are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVISATOS S. G. A., DENSTEDT O. F. Lactic dehydrogenase and DPN-ase activity of blood. Science. 1951 Sep 14;114(2959):281–283. doi: 10.1126/science.114.2959.281. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R., BARNET H. N. Changes in the phosphate compounds of the human red blood cell during blood bank storage. J Clin Invest. 1960 Jan;39:56–61. doi: 10.1172/JCI104026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959 Mar;234(3):459–465. [PubMed] [Google Scholar]
- BOYER P. D. Uses and limitations of measurements of rates of isotopic exchange and incorporation in catalyzed reactions. Arch Biochem Biophys. 1959 Jun;82(2):387–410. doi: 10.1016/0003-9861(59)90136-5. [DOI] [PubMed] [Google Scholar]
- Borgmann U., Moon T. W., Laidler K. J. Molecular kinetics of beef heart lactate dehydrogenase. Biochemistry. 1974 Dec 3;13(25):5152–5158. doi: 10.1021/bi00722a016. [DOI] [PubMed] [Google Scholar]
- Borgmann U., Moon T. W., Laidler K. J. Molecular kinetics of beef heart lactate dehydrogenase. Biochemistry. 1974 Dec 3;13(25):5152–5158. doi: 10.1021/bi00722a016. [DOI] [PubMed] [Google Scholar]
- Brindle K. M., Brown F. F., Campbell I. D., Grathwohl C., Kuchel P. W. Application of spin-echo nuclear magnetic resonance to whole-cell systems. Membrane transport. Biochem J. 1979 Apr 15;180(1):37–44. doi: 10.1042/bj1800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. F., Campbell I. D., Kuchel P. W., Rabenstein D. C. Human erythrocyte metabolism studies by 1H spin echo NMR. FEBS Lett. 1977 Oct 1;82(1):12–16. doi: 10.1016/0014-5793(77)80875-2. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M. The application of high resolution nuclear magnetic resonance to biological systems. Methods Biochem Anal. 1979;25:1–133. doi: 10.1002/9780470110454.ch1. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Rickert I., Beyer E. Stereoselective, SH-dependent transfer of lactate in mammalian erythrocytes. Biochim Biophys Acta. 1978 Feb 2;507(1):137–155. doi: 10.1016/0005-2736(78)90381-4. [DOI] [PubMed] [Google Scholar]
- Eby D., Kirtley M. E. Isolation and characterization of glyceraldehyde-3-phosphate dehydrogenase from human erythrocyte membranes. Arch Biochem Biophys. 1979 Dec;198(2):608–613. doi: 10.1016/0003-9861(79)90537-x. [DOI] [PubMed] [Google Scholar]
- Eckel R. E., Rizzo S. C., Lodish H., Berggren A. B. Potassium transport and control of glycolysis in human erythrocytes. Am J Physiol. 1966 Apr;210(4):737–743. doi: 10.1152/ajplegacy.1966.210.4.737. [DOI] [PubMed] [Google Scholar]
- FURFINE C. S., VELICK S. F. THE ACYL-ENZYME INTERMEDIATE AND THE KINETIC MECHANISM OF THE GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE REACTION. J Biol Chem. 1965 Feb;240:844–855. [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberman H. D. The fate of the alphah atom of L-lactate in perfused rat liver. Ann N Y Acad Sci. 1965 Jul 31;119(3):1070–1083. doi: 10.1111/j.1749-6632.1965.tb47463.x. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Steck T. L. Association of glyceraldehyde-3-phosphate dehydrogenase with the human red cell membrane. A kinetic analysis. J Biol Chem. 1980 Jul 10;255(13):6314–6321. [PubMed] [Google Scholar]
- MEHLER A. H. Kinetic properties of native and carboxy-peptidase-altered rabbit muscle aldolase. J Biol Chem. 1963 Jan;238:100–104. [PubMed] [Google Scholar]
- Minakami S., Yoshikawa H. Studies on erythrocyte glycolysis. II. Free energy changes and rate limitings steps in erythrocyte glycolysis. J Biochem. 1966 Feb;59(2):139–144. doi: 10.1093/oxfordjournals.jbchem.a128274. [DOI] [PubMed] [Google Scholar]
- NOVOA W. B., SCHWERT G. W. Lactic dehydrogenase. VIII. Binding of oxamate and of oxalate by enzyme-coenzyme complexes. J Biol Chem. 1961 Jul;236:2150–2153. [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of red cell glycolysis. The cause of triose phosphate accumulation. J Biol Chem. 1970 Aug 25;245(16):4009–4015. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Glycolysis-dependent exchange of diphosphopyridine nucleotide-3H in red blood cells and ascites cells. J Biol Chem. 1969 Mar 10;244(5):1114–1117. [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. Lactic dehydrogenase. III. Mechanism of the reaction. J Biol Chem. 1956 Nov;223(1):157–170. [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF BOVINE HEART AND RABBIT MUSCLE LACTATE DEHYDROGENASES. J Biol Chem. 1964 Nov;239:3901–3907. [PubMed] [Google Scholar]
- Simpson R. J., Brindle K. M., Brown F. F., Campbell I. D., Foxall D. L. A p.m.r. isotope-exchange method for studying the kinetic properties of dehydrogenases in intact cells. Biochem J. 1982 Mar 15;202(3):573–579. doi: 10.1042/bj2020573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Brindle K. M., Brown F. F., Campbell I. D., Foxall D. L. Studies of lactate dehydrogenase in the purified state and in intact erythrocytes. Biochem J. 1982 Mar 15;202(3):581–587. doi: 10.1042/bj2020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Brindle K. M., Brown F. F., Campbell I. D., Foxall D. L. Studies of pyruvate-water isotope exchange catalysed by erythrocytes and proteins. Biochem J. 1981 Feb 1;193(2):401–406. doi: 10.1042/bj1930401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON J. F., DARLING J. J., BORDNER L. F. ISOTOPE AND SOLVENT EFFECTS OF DEUTERIUM ON RABBIT-MUSCLE LACTATE DEHYDROGENASE. Biochim Biophys Acta. 1964 May 4;85:177–185. doi: 10.1016/0926-6569(64)90239-1. [DOI] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. IV. The influence of pH on the kinetics of the reaction. J Biol Chem. 1958 Apr;231(2):1065–1083. [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W., MILLAR D. B. Lactic dehydrogenase. VI. Fluorimetric measurements of the complex of enzyme and reduced diphosphopyridine nucleotide. J Biol Chem. 1959 May;234(5):1149–1154. [PubMed] [Google Scholar]
- Wang C. S. Inhibition of human erythrocyte lactate dehydrogenase by high concentrations of pyruvate. Evidence for the competitive substrate inhibition. Eur J Biochem. 1977 Sep;78(2):569–574. doi: 10.1111/j.1432-1033.1977.tb11770.x. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil G., Hoberman H. D. Rate of isotope exchange in enzyme-catalyzed reactions. Biochemistry. 1969 Jan;8(1):352–360. doi: 10.1021/bi00829a049. [DOI] [PubMed] [Google Scholar]
- Yagil G., Hoberman H. D. Rate of isotope exchange in enzyme-catalyzed reactions. Biochemistry. 1969 Jan;8(1):352–360. doi: 10.1021/bi00829a049. [DOI] [PubMed] [Google Scholar]


