Abstract
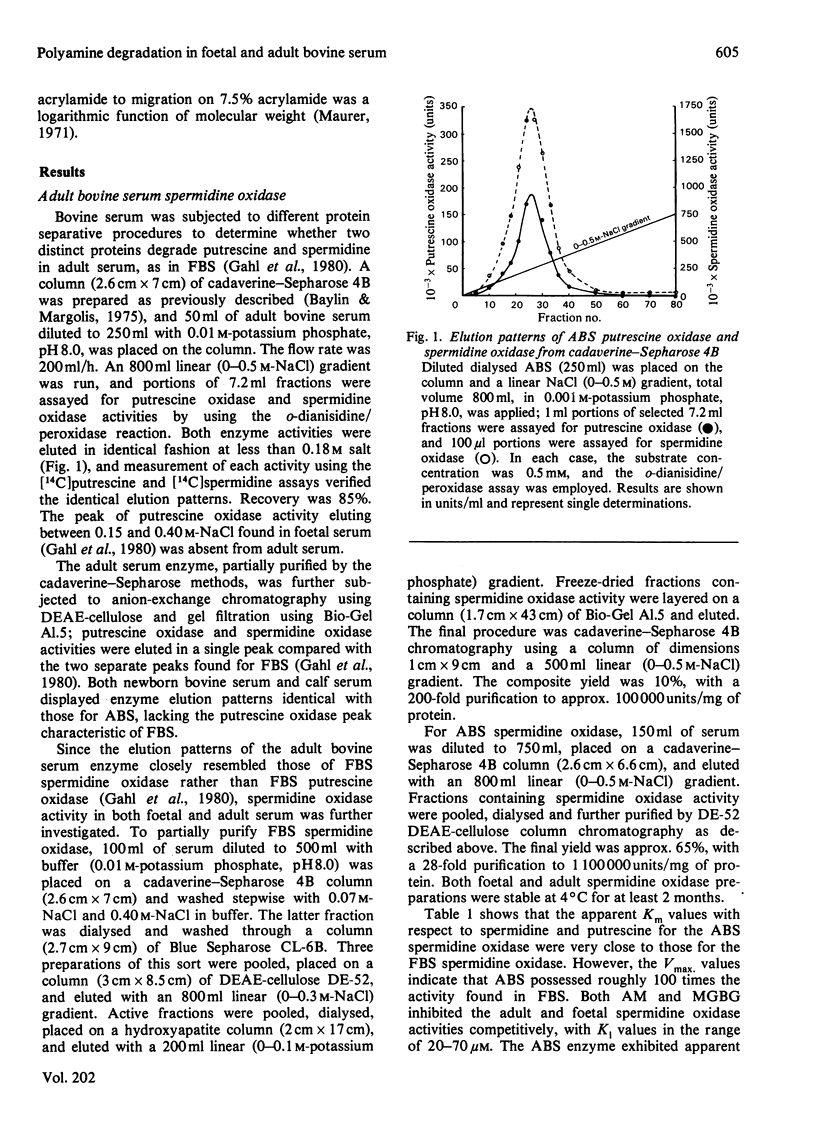
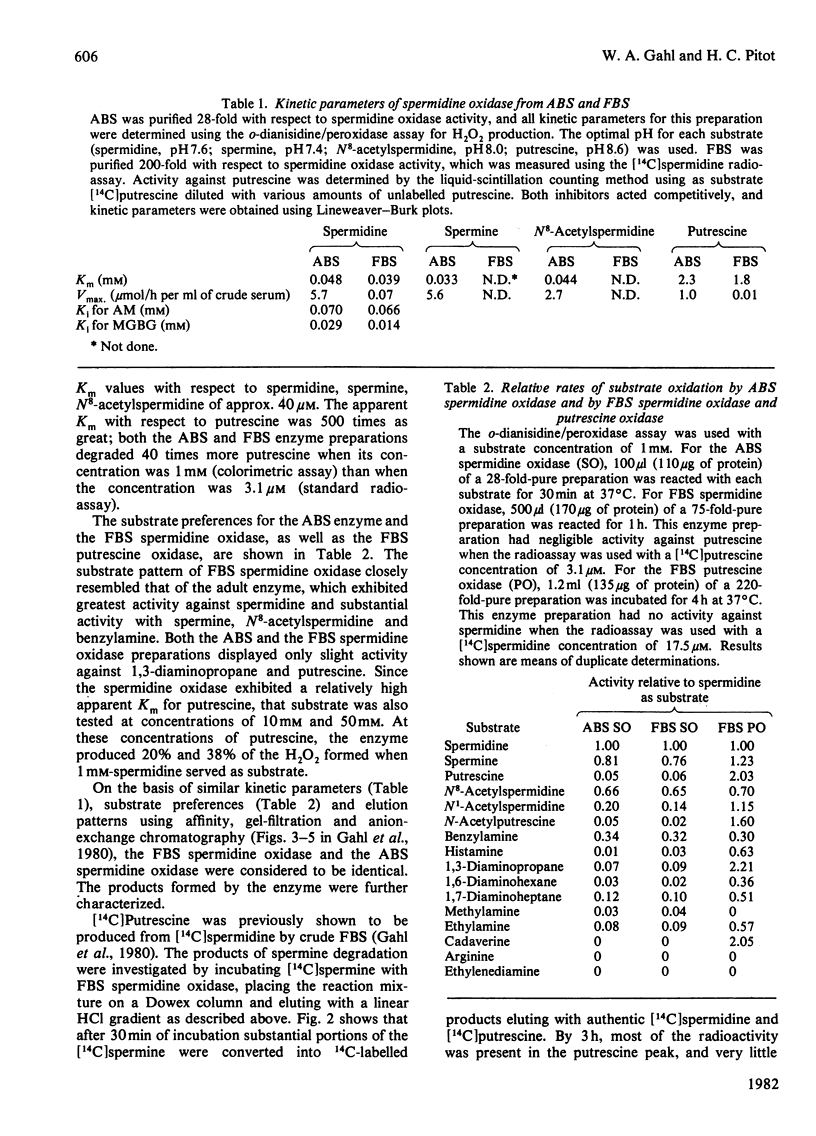
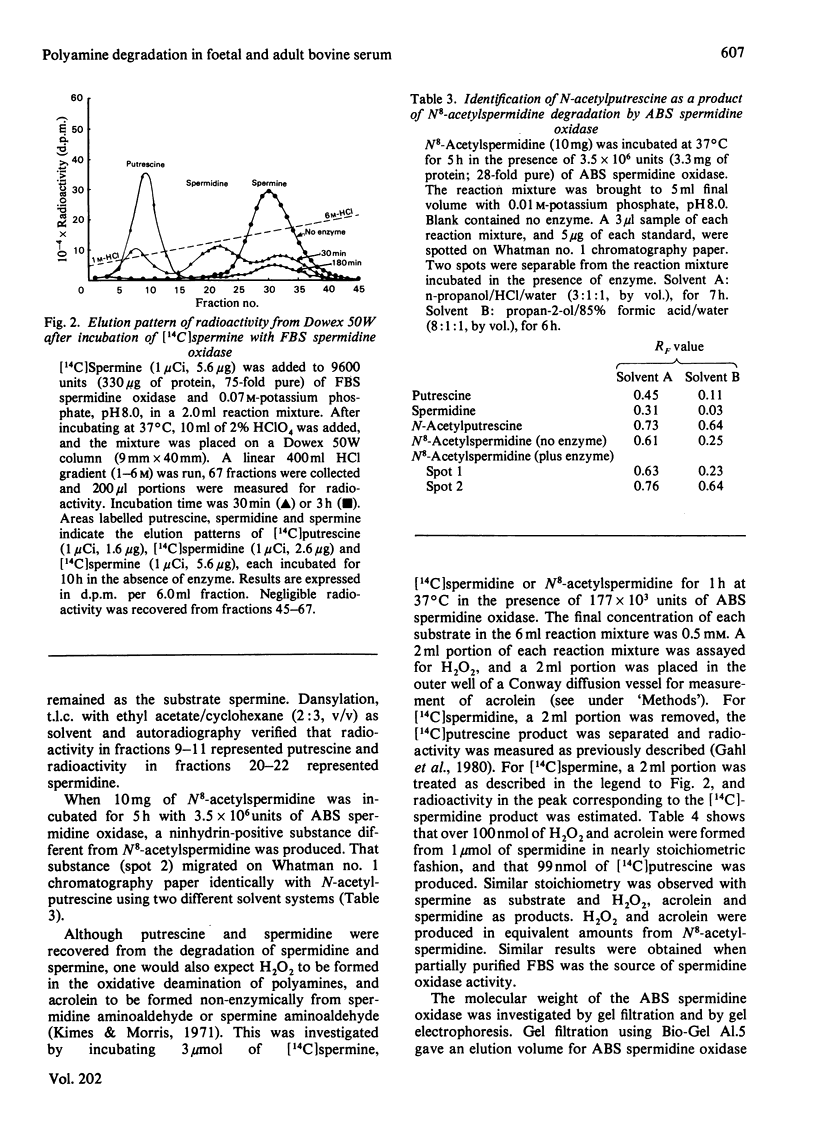
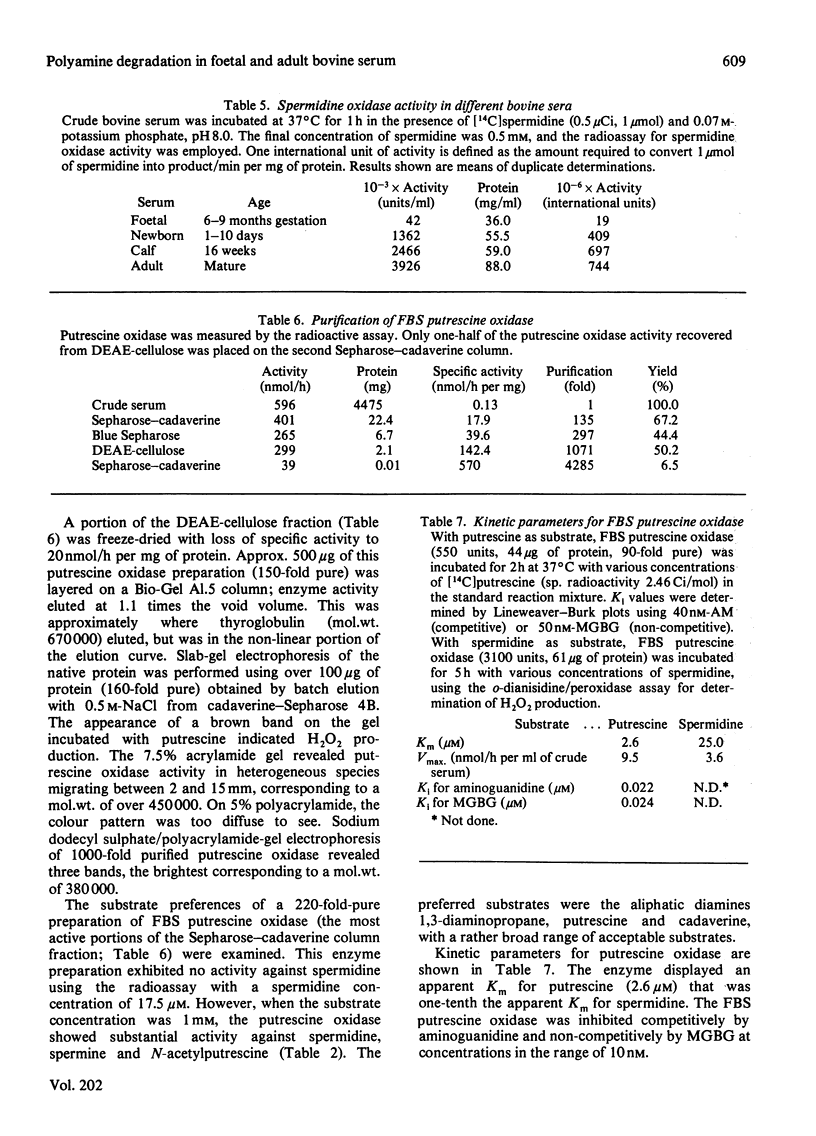
1. Using protein-separative chromatographic procedures and assays specific for putrescine oxidase and spermidine oxidase, adult bovine serum was found to contain a single polyamine-degrading enzyme with substrate preferences for spermidine and spermine. Apparent Km values for these substrates were approx. 40 microM. The apparent Km for putrescine was 2 mM. With spermidine as substrate, the Ki values for aminoguanidine (AM) and methylglyoxal bis(guanylhydrazone) (MGBG) were 70 microM and 20 microM respectively. 2. Bovine serum spermidine oxidase degraded spermine to spermidine to putrescine and N8-acetylspermidine to N-acetylputrescine. Acrolein was produced in all these reactions and recovered in quantities equivalent to H2O2 recovery. 3. Spermidine oxidase activity was present in foetal bovine serum, but increased markedly after birth to levels in adult serum that were almost 100 times the activity in foetal bovine serum. 4. Putrescine oxidase, shown to be a separate enzyme from bovine serum spermidine oxidase, was present in foetal bovine serum but absent from bovine serum after birth. This enzyme displayed an apparent Km for putrescine of 2.6 microM. The enzyme was inhibited by AM and MGBG with Ki values of 20 nM. Putrescine, cadaverine and 1,3-diaminopropane proved excellent substrates for the enzyme compared with spermidine and spermine, and N-acetylputrescine was a superior substrate to N1- or N8-acetylspermidine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M. M., Merdink J. L. Formation of N1-acetylspermidine in rat liver after treatment with carbon tetrachloride. Life Sci. 1981 May 4;28(18):2017–2023. doi: 10.1016/0024-3205(81)90649-4. [DOI] [PubMed] [Google Scholar]
- Alarcon R. A. Acrolein. IV. Evidence for the formation of the cytotoxic aldehyde acrolein from enzymatically oxidized spermine or spermidine. Arch Biochem Biophys. 1970 Apr;137(2):365–372. doi: 10.1016/0003-9861(70)90450-9. [DOI] [PubMed] [Google Scholar]
- Alarcon R. A. Fluorometric determination of acrolein and related compounds with m-aminophenol. Anal Chem. 1968 Sep;40(11):1704–1708. doi: 10.1021/ac60267a019. [DOI] [PubMed] [Google Scholar]
- Allen J. C., Smith C. J., Hussain J. I., Thomas J. M., Gaugas J. M. Inhibition of lymphocyte proliferation by polyamines requires ruminant-plasma polyamine oxidase. Eur J Biochem. 1979 Dec;102(1):153–158. doi: 10.1111/j.1432-1033.1979.tb06275.x. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKO H., HAWES R. Observations on spermine oxidase of mammalian plasma. J Physiol. 1959 Jan 28;145(1):124–131. doi: 10.1113/jphysiol.1959.sp006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B., Margolis S. Purification of histaminase (diamine oxidase) from human pregnancy plasma by affinity chromatography. Biochim Biophys Acta. 1975 Aug 26;397(2):294–306. doi: 10.1016/0005-2744(75)90119-9. [DOI] [PubMed] [Google Scholar]
- Blankenship J. Metabolic conversion of N1-acetylspermidine to putrescine by a subcellular fraction of rat liver. Proc West Pharmacol Soc. 1979;22:115–118. [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- Gahl W. A., Pitot H. C. Putrescine-oxidase activity in adult bovine serum and fetal bovine serum. In Vitro. 1979 Apr;15(4):252–257. doi: 10.1007/BF02618948. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Vale A. M., Pitot H. C. Separation of putrescine oxidase and spermidine oxidase in foetal bovine serum with the aid of a specific radioactive assay of spermidine oxidase. Biochem J. 1980 Apr 1;187(1):197–204. doi: 10.1042/bj1870197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther R. E., Glick D. Determination of histaminase activity in histologic samples and its quantitative distribution in intact human placenta and uterus. J Histochem Cytochem. 1967 Aug;15(8):431–435. doi: 10.1177/15.8.431. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Spermine oxidase: an amine oxidase with specificity for spermine and spermidine. J Exp Med. 1953 Mar;97(3):345–355. doi: 10.1084/jem.97.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O., Sarna G. P., Marton L. J., Omine M., Perry S., Russell D. H. Polyamine content of AKR leukemic cells in relation to the cell cycle. Cancer Res. 1973 Nov;33(11):2959–2964. [PubMed] [Google Scholar]
- Izard C., Libermann C. Acrolein. Mutat Res. 1978;47(2):115–138. doi: 10.1016/0165-1110(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Morris D. R. Preparation and stability of oxidized polyamines. Biochim Biophys Acta. 1971 Jan 1;228(1):223–234. doi: 10.1016/0005-2787(71)90562-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- Menashe M., Faber J., Bachrach U. Formulation of N-acetylputrescine and N1-acetylspermidine in cultured human lymphocytes. Biochem J. 1980 Apr 15;188(1):263–267. doi: 10.1042/bj1880263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUYAMA T., KOBAYASHI Y. Determination of diamine oxidase activity by liquid scintillation counting. Arch Biochem Biophys. 1961 Nov;95:242–250. doi: 10.1016/0003-9861(61)90141-2. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. Acetylation of spermidine in polyamine catabolism. Biochim Biophys Acta. 1980 Dec 1;633(2):181–190. doi: 10.1016/0304-4165(80)90404-3. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., BACHRACH U. IDENTIFICATION OF THE AMINOALDEHYDES PRODUCED BY THE OXIDATION OF SPERMINE AND SPERMIDINE WITH PURIFIED PLASMA AMINE OXIDASE. J Biol Chem. 1964 Jul;239:2194–2203. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. I. Purification, crystallization, and properties of plasma monoamine oxidase. J Biol Chem. 1962 May;237:1511–1516. [PubMed] [Google Scholar]
- Yasunobu K. T., Ishizaki H., Minamiura N. The molecular mechanistic and immunological properties of amine oxidases. Mol Cell Biochem. 1976 Oct 30;13(1):3–29. doi: 10.1007/BF01732392. [DOI] [PubMed] [Google Scholar]


