Abstract
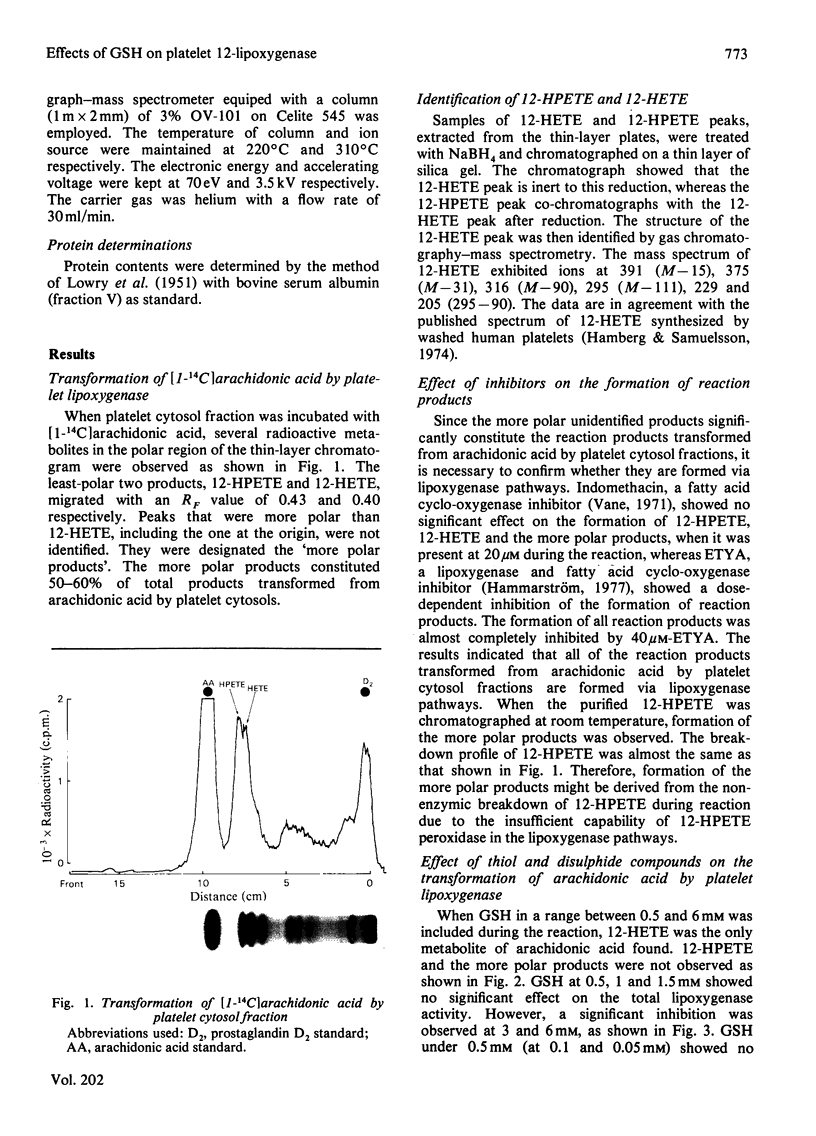
Arachidonic acid is converted into several more polar products in addition to 12-l-hydroperoxyeicosa-5,8,10,14-tetraenoic acid (12-HPETE) and 12-l-hydroxyeicosa-5,8,10,14-tetraenoic acid (12-HETE) by the cytosol fractions of rat platelets. The more polar products are formed via the lipoxygenase pathways in the same way as are 12-HPETE and 12-HETE, since their formation is not inhibited by indomethacin but by eicosa-5,8,11,14-tetraynoic acid (ETYA). The presence of 0.5–1.5mm-reduced glutathione (GSH) in the reaction mixture prevents the formation of the more polar products and produces 12-HETE as the only metabolite from arachidonic acid by the 12-lipoxygenase pathway. l-Cysteine has the same effect as GSH. However, oxidized glutathione (GSSG) and l-cystine are not able to prevent the formation of the more polar products. The results indicate that 12-HPETE peroxidase in the 12-lipoxygenase pathway is a GSH-dependent peroxidase and the more polar products might be formed from the non-enzymic breakdown of the primary 12-lipoxygenase product of 12-HPETE, owing to insufficient capability of the subsequent peroxidase system to completely reduce 12-HPETE to 12-HETE. Thus the presence of GSH in the reaction mixture offers a convenient and precise cell-free assay system for 12-lipoxygenase in rat platelets. Routine assays of 12-lipoxygenase are carried out in the presence of 1mm-GSH in the reaction mixture. The synthesis of 12-HETE by 12-lipoxygenase is linear during the first 4 min of incubation at 37°C, and has a pH optimum of 7.7. The 12-lipoxygenase reaches half-maximal activity at an arachidonate concentration of 20μm. Fractionation of cell homogenates indicates that the cytosol fraction possesses almost all the 12-lipoxygenase activity, whereas the microsomal fraction exhibits little enzyme activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant R. W., Bailey J. M. Altered lipoxygenase metabolism and decreased glutathione peroxidase activity in platelets from selenium-deficient rats. Biochem Biophys Res Commun. 1980 Jan 15;92(1):268–276. doi: 10.1016/0006-291x(80)91548-x. [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Bailey J. M. Isolation of a new lipoxygenase metabolite of arachidonic acid, 8. 11, 12-trihydroxy-5,9,14-eicosatrienoic acid from human platelets. Prostaglandins. 1979 Jan;17(1):9–18. doi: 10.1016/0090-6980(79)90071-6. [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Bailey J. M. Isolation of glucose-sensitive platelet lipoxygenase products from arachidonic acid. Adv Prostaglandin Thromboxane Res. 1980;6:95–99. [PubMed] [Google Scholar]
- Chang W. C., Murota S. I., Tsurufuji S. Role of prostaglandin E in carrageenin-induced inflammation in rats. Biochem Pharmacol. 1976 Sep 15;25(18):2045–2050. doi: 10.1016/0006-2952(76)90428-7. [DOI] [PubMed] [Google Scholar]
- Chang W. C., Murota S., Matsuo M., Tsurufuji S. A new prostaglandin transformed from arachidonic acid in carrageenin-induced granuloma. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1259–1264. doi: 10.1016/s0006-291x(76)80150-7. [DOI] [PubMed] [Google Scholar]
- Chang W. C., Murota S., Tsurufuji S. Thromboxane B2 transformed from arachidonic acid in carrageenin-induced granuloma. Prostaglandins. 1977 Jan;13(1):17–24. doi: 10.1016/0090-6980(77)90038-7. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S. Selective inhibition of platelet n-8 lipoxygenase by 5,8,11-eicosatriynoic acid. Biochim Biophys Acta. 1977 Jun 22;487(3):517–519. doi: 10.1016/0005-2760(77)90221-1. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Kerry P. J., Poyser N. L., Walker I. C., Wilson N. H. The identification of trihydroxyeicosatrienoic acids as products from the incubation of arachidonic acid with washed blood platelets. Prostaglandins. 1978 Oct;16(4):583–589. doi: 10.1016/0090-6980(78)90188-0. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Cuatrecasas P. Aspirin-like drugs interfere with arachidonate metabolism by inhibition of the 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid peroxidase activity of the lipoxygenase pathway. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3774–3778. doi: 10.1073/pnas.76.8.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Porter N. A., Cuatrecasas P. Arachidonate metabolism via lipoxygenase and 12L-hydroperoxy-5,8,10,14-icosatetraenoic acid peroxidase sensitive to anti-inflammatory drugs. Proc Natl Acad Sci U S A. 1980 Jan;77(1):308–312. doi: 10.1073/pnas.77.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F. F., McGuire J. C., Morton D. R., Pike J. E., Sprecher H., Kunau W. H. Inhibition of platelet arachidonic acid 12-lipoxygenase by acetylenic acid compounds. Prostaglandins. 1981 Feb;21(2):333–343. doi: 10.1016/0090-6980(81)90151-9. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]