Abstract
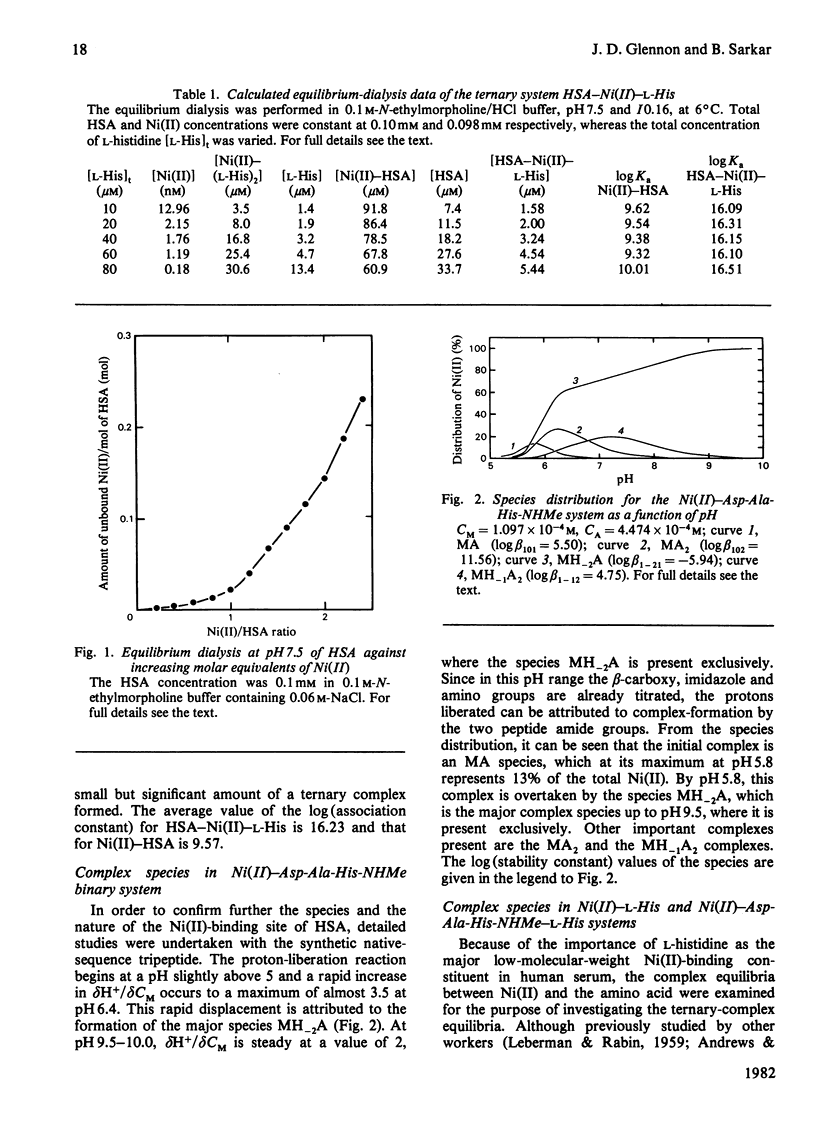
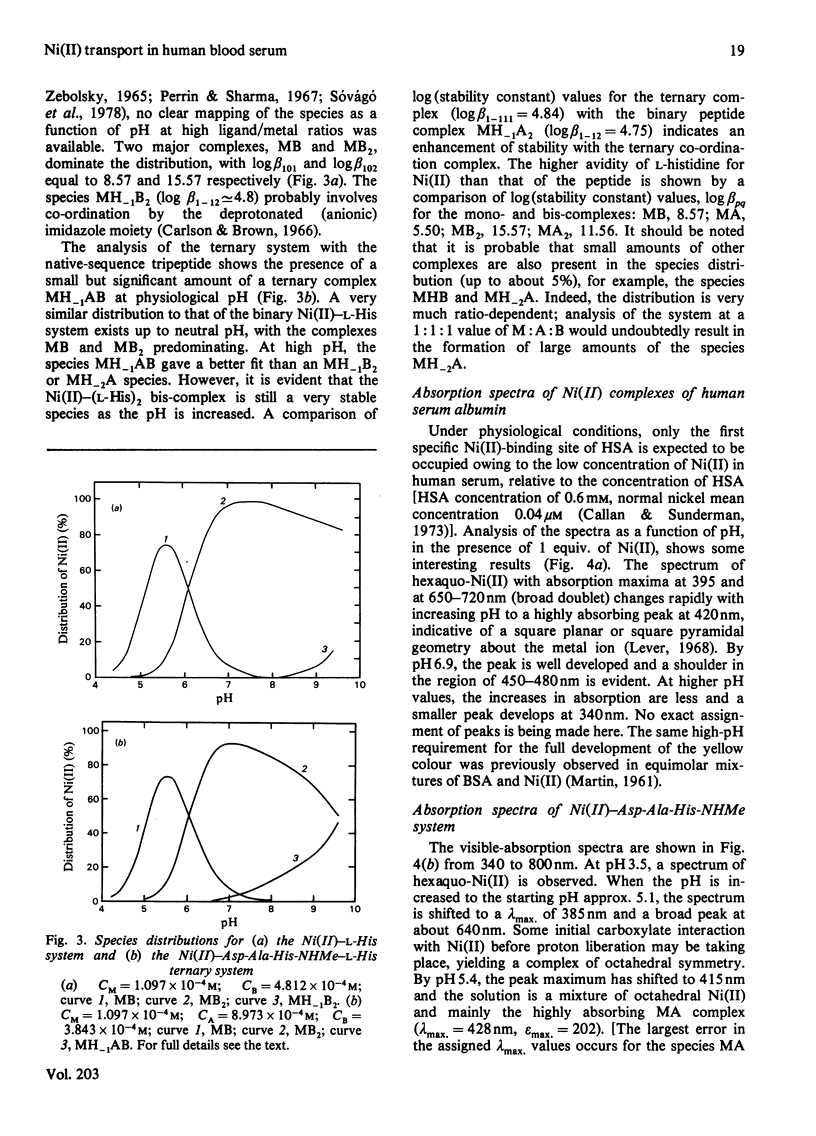
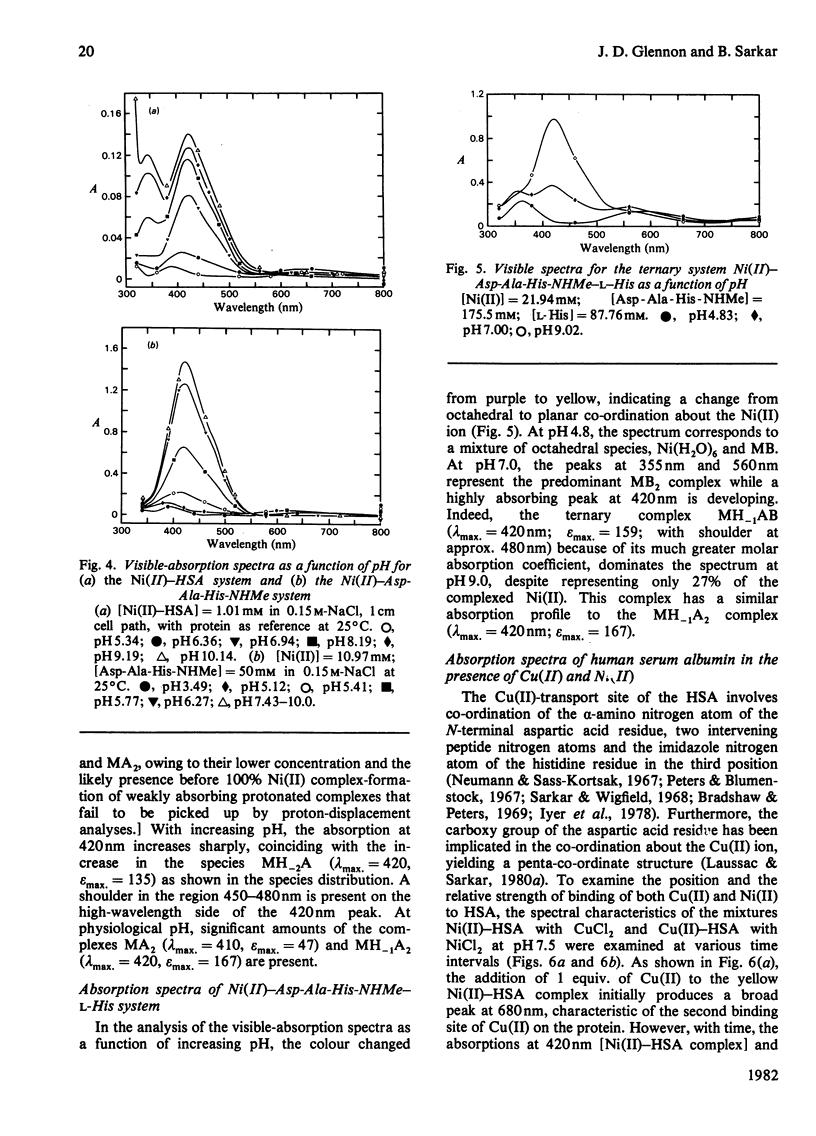
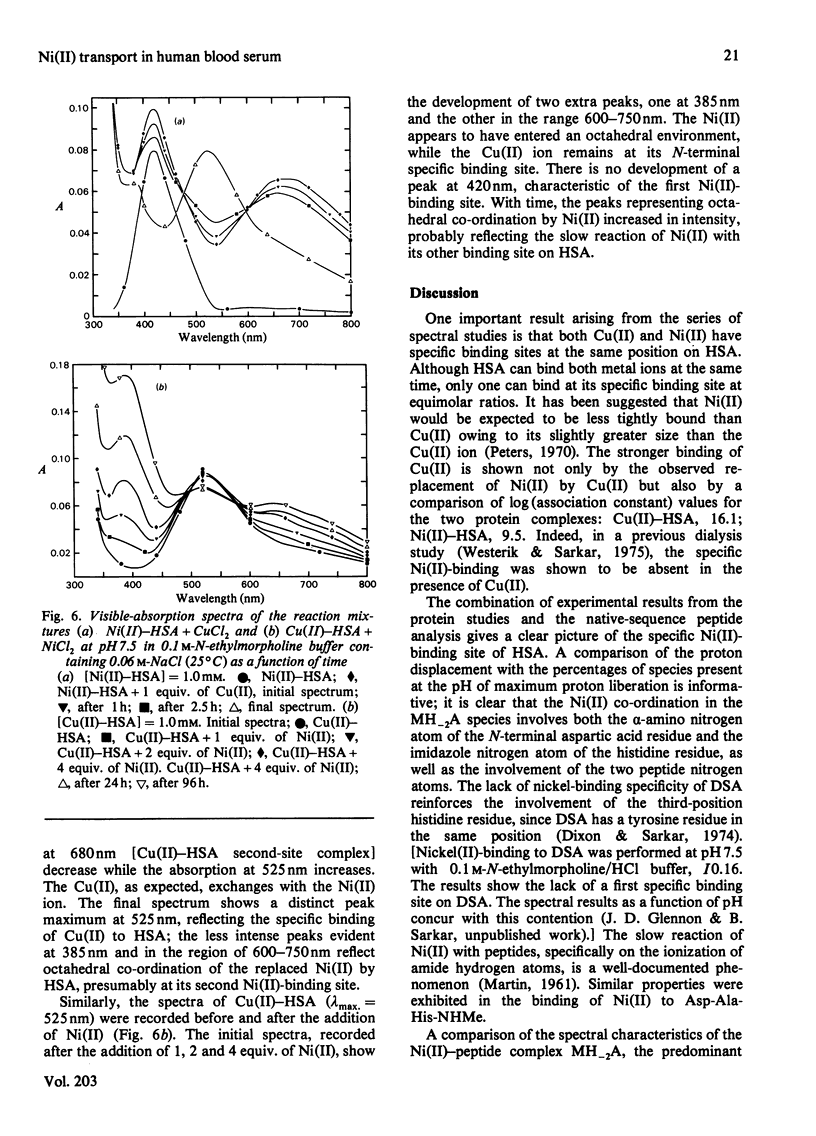
Detailed studies are reported on the Ni(II)-binding site of human serum albumin (HSA) and the results are compared with those obtained from the N-terminal native-sequence peptide, l-aspartyl-l-alanyl-l-histidine N-methylamide (Asp-Ala-His-NHMe). Equilibrium dialysis of HSA and Ni(II) in 0.1m-N-ethylmorpholine/HCl buffer, pH 7.53, demonstrates a specific Ni(II)-binding site on the protein. l-Histidine, the low-molecular-weight Ni(II)-binding constituent of human serum, is shown to have a greater affinity for Ni(II) than does HSA. A small but significant amount of ternary complex HSA–Ni(II)–l-histidine is also present in the equilibrium mixture containing the three components. The log (association constant) values for the binary and ternary Ni(II) complexes are 9.57 and 16.23 respectively. The complex equilibria between Asp-Ala-His-NHMe and Ni(II) have been investigated by analytical potentiometry in aqueous solution (0.15m-NaCl, 25°C). Several species, including MA, MA2, MH−2A, and MH−1A2 [where M and A represent Ni(II) ion and anionic peptide respectively], were detected in the system, MH−2A being the major complex species. Equilibrium studies involving Asp-Ala-His-NHMe, Ni(II) and l-histidine reveal the presence of a ternary complex MH−1AB (where B represents anionic l-histidine) at physiological pH. Detailed studies of visible-absorption spectra of HSA in the presence of Cu(II) and Ni(II) reveal that the two metal ions bind HSA at the same site. The visible-absorption spectrum of Ni(II)–HSA complex shows a highly absorbing peak at 420nm (εmax. = 137; with shoulder at 450–480nm) characteristic of a square planar or square pyramidal co-ordination arrangement about the metal ion. Similar visible-absorption characteristics were observed for the major species MH−2A in the Asp-Ala-His-NHMe–Ni(II) system (λmax. = 420nm; εmax. = 135; with shoulder at 450–480nm). The combination of experimental results from the protein studies and the peptide analyses provides strong evidence for the structure of the Ni(II)-binding site of HSA as one that involves the α-amino nitrogen atom, two deprotonated peptide nitrogen atoms, the imidazole nitrogen atom and the side-chain carboxy group of the aspartic acid residue. On the basis of the results obtained from the individual ternary systems involving protein and peptide, a mechanism for the transportation of Ni(II) in the serum is proposed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton D. W., Sarkar B. The absence of specific copper (II)-binding site in dog albumin. A comparative study of human and dog albumins. J Biol Chem. 1971 Aug 25;246(16):5040–5046. [PubMed] [Google Scholar]
- Bradshaw R. A., Peters T., Jr The amino acid sequence of peptide (1-24) of rat and human serum albumins. J Biol Chem. 1969 Oct 25;244(20):5582–5589. [PubMed] [Google Scholar]
- Callan W. M., Sunderman F. W., Jr Species variations in binding of 63 NI(II) by serum albumin. Res Commun Chem Pathol Pharmacol. 1973 Mar;5(2):459–472. [PubMed] [Google Scholar]
- Dixon J. W., Sarkar B. Isolation, amino acid sequence and copper(II)-binding properties of peptide (1-24) of dog serum albumin. J Biol Chem. 1974 Sep 25;249(18):5872–5877. [PubMed] [Google Scholar]
- Iyer K. S., Lau S. J., Laurie S. H., Sarkar B. Synthesis of the native copper(II)-transport site of human serum albumin and its copper(II)-binding properties. Biochem J. 1978 Jan 1;169(1):61–69. doi: 10.1042/bj1690061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. J., Kruck T. P., Sarkar B. A peptide molecule mimicking the copper(II) transport site of human serum albumin. A comparative study between the synthetic site and albumin. J Biol Chem. 1974 Sep 25;249(18):5878–5884. [PubMed] [Google Scholar]
- Lau S. J., Sarkar B. Ternary coordination complex between human serum albumin, copper (II), and L-histidine. J Biol Chem. 1971 Oct 10;246(19):5938–5943. [PubMed] [Google Scholar]
- Laussac J. P., Sarkar B. 13Carbon-nuclear magnetic resonance investigation of the Cu(II)-binding to the native sequence peptide representing the Cu(II)-transport site of human albumin. Evidence for the involvement of the beta-carboxyl side chain of aspartyl residue. J Biol Chem. 1980 Aug 25;255(16):7563–7568. [PubMed] [Google Scholar]
- Lucassen M., Sarkar B. Nickel(II)-binding constituents of human blood serum. J Toxicol Environ Health. 1979 Sep;5(5):897–905. doi: 10.1080/15287397909529799. [DOI] [PubMed] [Google Scholar]
- MARTIN R. B. Metal ion binding to peptides and proteins. Fed Proc. 1961 Sep;20(3):54–57. [PubMed] [Google Scholar]
- McNeely M. D., Nechay M. W., Sunderman F. W., Jr Measurements of nickel in serum and urine as indices of environmental exposure to nickel. Clin Chem. 1972 Sep;18(9):992–995. [PubMed] [Google Scholar]
- Neumann P. Z., Sass-Kortsak A. The state of copper in human serum: evidence for an amino acid-bound fraction. J Clin Invest. 1967 Apr;46(4):646–658. doi: 10.1172/JCI105566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F. H., Myron D. R., Givand S. H., Zimmerman T. J., Ollerich D. A. Nickel deficiency in rats. J Nutr. 1975 Dec;105(12):1620–1630. doi: 10.1093/jn/105.12.1620. [DOI] [PubMed] [Google Scholar]
- Nomoto S., McNeely M. D., Sunderman F. W., Jr Isolation of a nickel alpha 2-macroglobulin from rabbit serum. Biochemistry. 1971 Apr 27;10(9):1647–1651. doi: 10.1021/bi00785a023. [DOI] [PubMed] [Google Scholar]
- Onkelinx C., Becker J., Sunderman F. W., Jr Compartmental analysis of the metabolism of 63Ni(II) in rats and rabbits. Res Commun Chem Pathol Pharmacol. 1973 Sep;6(2):663–676. [PubMed] [Google Scholar]
- Peters T., Jr, Blumenstock F. A. Copper-binding properties of bovine serum albumin and its amino-terminal peptide fragment. J Biol Chem. 1967 Apr 10;242(7):1574–1578. [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Clin Chem. 1970;13:37–111. doi: 10.1016/s0065-2423(08)60385-6. [DOI] [PubMed] [Google Scholar]
- Sarkar B., Wigfield Y. Evidence for albumin--cu(II)--amino acid ternary complex. Can J Biochem. 1968 Jun;46(6):601–607. doi: 10.1139/o68-092. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr A review of the metabolism and toxicology of nickel. Ann Clin Lab Sci. 1977 Sep-Oct;7(5):377–398. [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Decsy M. I., McNeely M. D. Nickel metabolism in health and disease. Ann N Y Acad Sci. 1972 Jun 28;199:300–312. doi: 10.1111/j.1749-6632.1972.tb46465.x. [DOI] [PubMed] [Google Scholar]


