Abstract
Pain serves as a vital innate defense mechanism that can significantly impact an individual’s quality of life. Understanding the physiological effects of pain well plays an important role in developing novel pain treatments. Nociceptor neurons play a key role in pain and inflammation. Interactions between nociceptors and the immune system occur both at the site of injury and within the central nervous system. Modulating chemical mediators and nociceptor activity offers promising new approaches to pain management. Essentially, the sensory nervous system is essential for modulating the body’s protective response, making it critical to understand these interactions to discover new pain treatment strategies. New innovations in neuromodulation have led to alternatives to opioids individuals with chronic pain with consequent improvement in disease-based treatment and nerve targeting. New neural targets from cellular and structural perspectives have revolutionized the field of neuromodulation. This narrative review aims to elucidate the mechanisms of pain transmission and processing, examine the characteristics and properties of nociceptors, and explore how the immune system influences pain perception. It further provides an updated overview of the physiology of pain and neuromodulatory mechanisms essential for managing acute and chronic pain. We assess the current understanding of different pain types, focusing on key molecules involved in each type and their physiological effects. Additionally, we compare painful and painless neuropathies and discuss the neuroimmune interactions involved in pain manifestation.
Keywords: neurons, nociceptive pain, neuropathic pain, nociplastic pain, physiological effects, pain pathways, sensitization
Introduction
The nervous system is an incredibly intricate portion of human anatomy that serves four major functions: motion, sensation, association, and control of homeostasis.1 It is broadly characterized into the central nervous system (CNS) and the peripheral nervous system (PNS); both of which play roles in pain pathways.
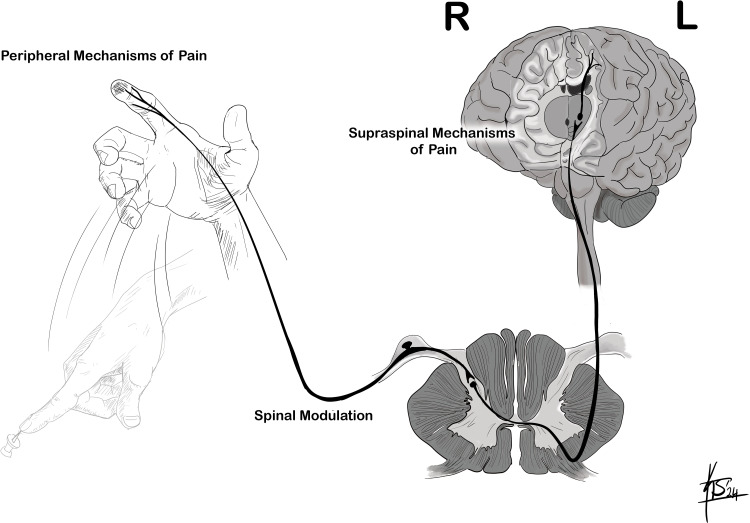
Pain systems are neural circuits responsible for the sensation and perception of pain, as well as the body’s response to pain. They consist of peripheral neurons (primary afferent neurons) with sets of peripheral receptive elements (nociceptors) that connect in the dorsal horn of the spinal cord with central neuronal relay pathways (secondary afferent neurons) and integrative neurons that modulate nociceptive signals through excitatory or inhibitory influences at various levels of the neuraxis.2 Nociception encompasses four stages: transduction, transmission, modulation, and perception. During transduction, a physical or chemical stimulus is converted into an electrical signal that can then be transmitted. Transmission refers to the movement of this electrical activity through the nervous system. Modulation is the alteration of neuronal activity through the pathways of transmission. Lastly, perception is when somatosensory transmission results in the subjective experience of pain.3
When there is traumatic injury and/or persistent inflammatory changes, this can affect the normal pain pathways, which can result in chronic pain. The progression from acute pain to a chronic pain state involves multilevel changes, including the primary sensory neuron, spinal cord, and brain.4 At the initial site of injury inflammatory mediators are secreted that stimulate nociceptor activity, this is sometimes referred to as the “Inflammatory soup”. The “inflammatory soup” is made up of peptides, neurotransmitters, lipid, and neuropeptides that can either activate nociceptors or lower their threshold to activation leading to increased activation of the a-delta and c fibers and peripheral sensitization occurs.5
Evolution of Neuromodulation to the Current Devices and Waveforms
The application of electrical stimulation for treating chronic pain has been observed since the first century C.E. when it was observed that contact with electrical fish could relieve gout pain.6 The modern application of neuromodulation for chronic pain began in the 1950s with deep brain stimulation and gained further momentum in the 1960s with the publication of Melzack and Wall’s gate control theory.6,7
Neuromodulation is a broad term that describes the modification of neurological function through delivery of a stimulus to specific targets.8,9 The modalities can be electrical, magnetic, chemical, and/or genetic. In chronic pain management, neuromodulation involves the use of non-invasive, minimally invasive, and/or surgical electrical therapies to modulate or alter the perception of pain.6 Commonly applied techniques of neuromodulation in the treatment of chronic pain involve spinal cord stimulation, peripheral nerve stimulation, and deep brain stimulation.10
Current neuromodulation devices can be broadly classified as open-loop stimulation or closed-loop stimulation. Open-loop systems provide a train of impulses to anatomic targets continuously or on a fixed cycle and can be activated/deactivated and adjusted by either the patient or an operator but do not adjust automatically.8,11 Closed-loop systems adjust their stimulation automatically, in real time, according to some form of clinically relevant physiologic data, such as postural changes affecting the distance of the epidural electrodes to the spinal cord detected through evoked compound action potentials.8,11
Traditional spinal cord stimulation, which was the standard treatment for the first four decades of therapy, is open-loop, low-frequency, and a tonic form of stimulation that is delivered at frequencies ranging from tens to hundreds of hertz, at an intensity that induces a paresthesia yet does not elicit a motor response or cause discomfort.11 Paresthesia coverage historically was thought to be of the utmost importance in predicting patient pain relief as documented by Barolat; however, current research suggests that with certain programs and frequency settings, paresthesia coverage is not required.12 Burst stimulation was initially introduced for chronic neuropathic pain treatment in 2010. It involves delivering a series of high-frequency pulses periodically at a lower rate.11,12 A meta-analysis comparing burst stimulation to tonic stimulation for the management of chronic low back pain demonstrated superiority of burst stimulation over tonic stimulation.13 This meta-analysis design represents the highest level of peer-reviewed evidence and suggests reproducibility.
Sub-perception stimulation, also referred to as paresthesia-free stimulation, is stimulation that does not reach the threshold of perception (paresthesia), due to low amplitude, waveform design, ultra-low frequency or high-frequency.11 High-density or high-dose stimulation is a type of stimulation that is meant to provide a higher charge per second (dose) at a relatively high frequency; typically, with an amplitude that has been adjusted below that which would elicit a paresthesia.11,12 High-frequency therapy, 10-kHz, utilizes a higher frequency with a shorter pulse-width to obtain pain relief without eliciting any paresthesia. This form of therapy is paresthesia independent and thus lead placement is based solely on anatomical landmarks,12 although some patients do complain of uncomfortable stimulation at very high amplitudes.
Basic Mechanisms of Pain
Types of Neurons
Neurons are found all throughout the human body and serve a multitude of different sensory and motor functions. Neurons consist of similar structures: soma (cell body), axon, and dendrites. The three major types of neurons are sensory, interneurons, and motor. Sensory neurons involved in detecting physical and chemical noxious stimuli are called nociceptors. They consist of cell bodies that arise in the dorsal root ganglion and axonal processes that travel to the periphery or centrally into the spinal cord. Nociceptors can be activated by temperature, mechanical, and chemical stimuli.
Types of Axons
Axons consist of various nerve fibers that conduct action potentials from the terminal dendrites to the axonal terminals or from one neuron to another.2 Nociceptors are primarily characterized by their axonal-free endings, fiber diameter or conduction velocity.14 The two primary afferent neurons are Aδ fibers and C fibers. Type Aδ fibers are medium diameter myelinated afferents that convey acute, well-localized, sharp pain. They are the smallest myelinated fibers, with a diameter of 2–5 µm and a conduction velocity of 30 m/s. C fibers are unmyelinated and transmit poorly localized stimuli, with a slower conduction velocity of 2 m/s. Type Aδ fibers are primarily thermal and mechanical nociceptors, whereas type C fibers are polymodal and respond to thermal, mechanical, and chemical stimuli.
Action Potentials in Neurons
An action potential is a sequence of changes in the voltage across a membrane of a cell that is created by a depolarizing current. When Na+ ions enter the cell, they result in increase in the voltage in the cell, taking it from its resting potential to its threshold potential. Once the threshold potential has been reached, complete depolarization occurs. Once peak potential has been reached, the Na+ channels return to their resting state and K+ channels are activated, resulting in hyperpolarization.15 The speed of transmission of the action potential is directly correlated to the diameter of the axons and the presence of myelin.16 Nodes of Ranvier, or gaps in the myelin sheath, allow in an increase in the conduction velocity. Action potentials ultimately allow for release of terminal neurotransmitters.
Synaptic Transmission in the Synaptic Cleft
Chemical synapses are mediated through the secretion of neurotransmitters at the level of the synaptic cleft.17 Synaptic signals are received by the terminal dendrites and soma and are transmitted within the neuron via axons. Depolarization results in an influx of calcium ions into the axon terminal, which bind to the calcium-sensing proteins that directly interact with soluble-ethylmaleimide-sensitive-factor activating protein receptor (SNARE) proteins. These are responsible for the fusion of the synaptic vesicles, which contain neurotransmitters, to the presynaptic axon terminal membrane, resulting in release of the neurotransmitters into the synaptic cleft. The neurotransmitters then diffuse across the synaptic cleft and bind to specific ion channels that are located on the postsynaptic neuron’s membrane.
Routes of Pain Transmission
Transmission is one of the three fundamental steps in the perception of pain. Transmission refers to the relay of the nociceptive signals from the periphery to the spinal cord. This begins when the primary afferent nociceptors release neurotransmitters in the dorsal horn, which activate second-order neurons. The second-order neurons cross contralaterally at the level of the spinal cord and ascend as the spinothalamic tract to project into the brain stem and thalamus.18 Third-order neurons are located at the level of the thalamus and project impulses to the primary sensory cortex for further processing and pain perception.
Types of Pain
Nociceptive Pain
Nociceptive input injury activates peripheral Transient Receptor Potential (TRP) nociceptors, leading to depolarization and action potential propagation to the dorsal root ganglia.16 Here, pseudounipolar neurons (first-order) receive this input and send axonal processes to the spinal cord’s dorsal horns.19 These axons synapse in the spinal gray matter, affecting second-order neurons, which cross the spinal cord and ascend in the anterolateral aspects of the spinal cord (becoming one of the tracts comprising the anterolateral pathways) towards the medulla oblongata, pontine and midbrain tegmentum. Eventually, these neurons synapse the thalamus’s ventral posterior nucleus (VPL). From the VPL, the information traveling in the spinothalamic tract is conveyed via thalamic somatosensory radiations (the third-order neurons of this pathway) to the primary somatosensory cortex in the postcentral gyrus (Brodmann areas 3, 1, 2).20 However, it is important to highlight that pain and temperature sensation for the face as carried by an analogous pathway, the trigeminothalamic tract. The Anterolateral Pathways are composed mainly by three tracts: spinothalamic (previously described), spinoreticular tracts, and spinomesencephalic tract. The spinothalamic and spinoreticular tracts together convey emotional and arousal aspects of pain; the spinoreticular tract terminates in the medullary–pontine reticular formation, projecting to intralaminar thalamic nuclei project diffusely to the entire cerebral cortex and is involved in the behavioral arousal caused by the nociceptive stimulus and spinomesencephalic (participates in central modulation of pain).21
Modulatory Output
Pain modulation represents a complex process, encompassing interactions among local neural circuits in the spinal cord’s dorsal horn and extensive modulatory inputs from farther regions (Figure 1). The Gate Control Theory posits that sensory signals from wide-diameter, non-painful A-β fibers are key in diminishing pain transmission at the dorsal horn of the spinal cord.22 This concept is utilized in therapeutic approaches like transcutaneous electrical nerve stimulation (TENS), where A-β fibers are stimulated to mitigate chronic pain.17
Figure 1.
Route of pain transmission.
The periaqueductal gray stands out as a pivotal component in pain modulation, receiving signals from the hypothalamus, amygdala, and cortex. It significantly contributes to the inhibition of pain transmission at the dorsal horn, mainly through a pathway at the pontomedullary junction, particularly within the rostral ventral medulla (RVM).23 The RVM is notable for its serotonergic (5-HT) neurons, originating from the raphe nuclei, which extend to the spinal cord and play a role in pain modulation.23 Furthermore, it transmits inputs via substance P to the locus ceruleus, thereby affecting noradrenergic pathways that regulate pain in the spinal cord’s dorsal horn. Histamine also plays a role in this complex modulation mechanism, particularly through its action on H3 receptors. Opiate medications like morphine are key players in pain modulation. Enkephalin and dynorphin are primarily concentrated in the periaqueductal gray, RVM, and spinal cord dorsal horn, while β-endorphin-containing neurons are predominantly located in regions of the hypothalamus that project to the periaqueductal gray.23,24 This intricate network of pathways and modulators underlines the complexity of pain perception and its regulation in the human body.
Neuropathic Pain
Neuropathic pain is defined as pain resulting from direct damage to the somatosensory nervous system.25 The symptoms of neuropathic pain can be classified as either positive or negative sensory symptoms. Positive symptoms, arising from nociceptive hypersensitivity, include allodynia, hyperalgesia, and paresthesia.26 Conversely, negative symptoms, which result from afferent neuronal injury, lead to incomplete input to the nervous system and are characterized by hypoalgesia and hypoesthesia. Diagnosing neuropathic pain involves a comprehensive history and physical examination, and confirmation can be achieved through histological, electrophysiological, and structural imaging tests.27
Treatment of neuropathic pain typically begins with pharmacotherapy, which includes tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and calcium channel ligands.28–31 If initial therapy fails, interventional methods can be a viable treatment approach.9,32 These procedures include steroid injections/neural blockade, neuroablative procedures, transcranial/epidural stimulation, spinal cord stimulation, deep brain stimulation, and percutaneous stimulation.33 Additionally, emerging methods being explored include gene therapy, strategies targeting ion channels, and the use of optogenetics and chemogenetics.34
Inflammatory Pain
Inflammatory pain is characterized by heightened sensitivity due to the inflammatory response associated with tissue damage.35 This response is triggered by extracellular inflammatory mediators released from the surrounding damaged tissues and nociceptive fibers.
These mediators encompass a variety of substances that are released upon cell damage, as well as those that are generated following tissue injury. Proinflammatory substances include reactive oxygen species, protons, kinins, prostanoids, bradykinin, adenosine triphosphate, serotonin, histamine, cytokines, neurotrophins, and neuropeptides.26
Mechanisms of Hyperalgesia and Allodynia
Hyperalgesia and allodynia are positive sensory neuropathic symptoms resulting from nociceptor hypersensitivity.23 The IASP Task Force defines hyperalgesia as increased pain sensitivity,36 while allodynia is characterized as pain due to a non-nociceptive stimulus.36 The mechanisms underlying hyperalgesia and allodynia include the upregulation of nociceptive pathways and a failure to inhibit these pathways.
Multiple mechanisms have been identified concerning the upregulation of the nociceptive pathway, with the two primary ones being peripheral and central sensitization.37 Peripheral sensitization occurs due to alterations in the unmyelinated C and myelinated A-delta fibers, leading to hypersensitivity. These changes at the molecular and cellular levels cause neurons to become abnormally sensitive to noxious stimuli.38 Central sensitization involves modifications within the spinal cord.27 Electrical stimulation of sensory nerves at C fiber intensity leads to the spinal release of amino acids, including glutamate and neuropeptides, such as substance P, and neurotrophic factors, such as brain-derived neurotrophic factor, culminating in spinal upregulation.
The failure of the nociceptive system’s inhibitory mechanism, leading to hyperalgesia, is referred to as attenuation. Attenuation’s role in inhibition involves both pre and postsynaptic inhibition of nociceptive spinal dorsal horn neurons.36 Conversely, the failure of the inhibitory mechanism in the nociceptive system that results in allodynia is termed separation. The inhibition role of separation entails the suppression of excitatory interneurons, which are responsible for establishing somatotopic borders.36
Peripheral Sensitization
Initially characterized in the 1970’s by Perl et al, peripheral sensitization is defined as increased responsiveness with reduced thresholds of nociceptive neurons to peripheral stimulation.39–41 This phenomenon typically occurs after peripheral nerve injury, tissue injury, and inflammation.41 Deemed primary hyperalgesia, it is believed that injury results in lowered thresholds of primary afferents in response to noxious stimuli, nerve fiber enhanced response to stimuli, and ultimately increase innervation of the injured site by adjacent nerve fibers.42 Post-translational changes to various ion channels have been found to occur at the site of injury resulting in alterations of nerve fiber depolarization with associated changes in gene expression and protein expression.41,43 These changes and associated alterations have been suggested to lead to peripheral sensitization.
Several ion channels have been suggested to be affected by peripheral sensitization, including voltage-gated transient receptor potential vanilloid (TRPV) channels, voltage gated sodium channels, and voltage gated calcium channels. TRPV1 has been seen to be an important channel for pain sensitization and several chronic pain states.44–46 TRPV1 activation allows for increases in intracellular sodium and calcium, triggering membrane action potentials mediating secondary messengers, which subsequently upregulates TRPV1 expression and sensitivity resulting in mechanical and thermal hypersensitivity.41,45,47 Many animal studies have shown that TRPV1 channel knockout mice do not develop the typical thermal and mechanical hyperalgesia after peripheral neurologic insult compared to TRPV1 channel preserved mice. TRPV1 has also been suggested as being necessary to maintain the hyperalgesia state.48,49 In humans, reports of malfunctional TRPV1 resulted in elevated pain thresholds during pain phenotyping experiments.50
Voltage gated sodium channels are expressed in numerous cell membranes for action potential regulation. Several channels including NaV1.1, NaV1.6, NaV1.7, NaV1.8, and NaV1.9 are expressed in a variety of sensory neurons.41 NaV1.7 is of particular interest as it is highly expressed in the dorsal root ganglion, peripheral sensory nerves, and sympathetic neurons.51,52 In animal studies, the loss of function of NaV1.7 resulted in mice with reduced hypersensitivity to pain meanwhile increased NaV1.7 function may be associated with the development of peripheral neuropathic states such as chemotherapy induced peripheral neuropathy and erythromelalgia.18,53,54
Intracellular calcium is an important cellular response leading to neurotransmitter release causing membrane depolarization and act as secondary messengers.55 Two types of calcium channels exist, high voltage (L, N, P, Q, and R type) and low voltage (T type), both of which have been implicated in the pain processing pathways.56 Animal studies with blockade of high voltage calcium channels have shown to decrease subjective pain and hyperalgesia.57–59 Similarly, low voltage calcium channels are important for regulation of nerve fiber excitability. Increased excitability of these channels may help promote the peripherally sensitized state and certain calcium channels are currently investigated as viable drug targets for treatment of chronic pain.60–62 It is theorized that alterations in ion channel regulation ultimately results in protein and neurotransmitter modulation causing the peripherally sensitized state.
Central Sensitization
Central sensitization is characterized as the enhancement in the function of neurons and circuits in nociceptive pathways caused by increases in membrane excitability and synaptic efficacy as well as to reduced inhibition and is a manifestation of the remarkable plasticity of the somatosensory nervous system in response to activity, inflammation, and neural injury.63
The concept was hypothesized to explain neuroplastic changes of synaptic function within the central nervous system after the discovery that nociceptive peripheral nerve injuries could sensitize peripheral nociceptive terminals.39,40,64–66 Central sensitization is implicated in multiple rheumatologic and chronic pain conditions including fibromyalgia, arthritis (osteo and rheumatoid), Ehlers-Danlos syndrome, upper extremity tendinopathies, headache, and spine pain.39,67–69 Unique from windup, central sensitization results in lower nociceptive input to create and/or sustain neural activation or potentially allow for amplified responses to non-stimulated or non-nociceptor fibers after cessation of conditioning stimuli.39,70
Several theories have been suggested to explain the pathophysiology of central sensitization though the exact mechanism is unknown. Descending (neural inhibitory) and ascending (neural activating) pathways are often discussed when assessing neuroplastic changes resulting in augmented sensory and pain processing.71
Glutamate is an important neurotransmitter implicated in the development of central sensitization. Glutamate binds to amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), N-methyl-D-Aspartate (NMDA), and several metabotropic glutamate receptor subtypes (mGluR) in the post-synaptic neurons of the dorsal horn.63,72,73 Activation of the NMDA receptor is essential for initiating and maintaining central sensitization as NMDA receptor antagonism reverses and prevents nociceptive neuronal hyperexcitability.74,75 NDMA activation allows for increased intracellular calcium, inducing intracellular kinases that maintain central sensitization by increasing phosphorylation of the AMPA and NDMA receptors. This results in post-translational modification changing the activity level and trafficking of these receptors and other channels that result in central sensitization.63 Aside from glutamate, substance P and brain derived neurotrophic factor have also been implicated contributing to central sensitization by enhancing C fiber evoked responses.63,76
The anterior cingulate cortex (ACC), periaqueductal grey, subnucleus reticularis dorsalis (SRD), and the rostral ventromedial medulla (RVM) play a large part in the descending pathway.71,77 The RVM and the nuclear raphe magnus contains serotonergic and GABAergic neurons, while dorsolateral posterior tegmentum (DLPT) contains noradrenergic neurons that inhibit neurotransmission at the dorsal horn. Patients with central sensitization have been found to have disrupted descending pathway processing resulting in alterations in serotonin and noradrenergic secretion.78 Patients with fibromyalgia have lower levels of noradrenergic and serotonergic neurotransmitters in bodily fluids as well as fewer opioid receptors but with excess of excitatory neurotransmitters in the pain modulating centers of the brain compared to healthy individuals.79 Functional MRI studies of patients with fibromyalgia and central sensitization have shown significant gray matter reduction in the ACC and prefrontal cortex with otherwise global preservation of gray matter with greater gray matter reduction in patients with long-standing fibromyalgia.68,80 These collective changes in the ascending and descending pain processing pathways have been suggested to contribute to the development of central sensitization.
Neurogenic-Induced Inflammation
Neurogenic-induced inflammation is activation of inflammatory pathways in the Peripheral Nervous System (PNS) and Central Nervous System (CNS) which lead to classical inflammatory reactions and is implicated in chronic pain syndromes. Chronic activation of this inflammatory pathway can lead to peripheral and central sensitization.81 Nociceptors, specified cells which are located in the periphery and, when activated, convey pain to the CNS, can be activated through inflammatory cytokines and neuropeptides such as substance P, calcitonin gene-related protein (CGRP), and neuropeptide Y. Therefore, neurogenic inflammation can directly lead to pain. There is a delicate balance in regards to neuroinflammation since it is appropriately activated in response to deleterious insults on the body (infection, traumatic brain injury, and autoimmune diseases)82 however, this inflammation can persist and lead to chronic pain.83 Chronic neuroinflammation is also seen in chronic inflammatory diseases such as asthma, migraine headaches, psoriasis, and complex regional pain syndrome (CRPS).81 There is a distinction between central and peripheral sensitization, and neurogenic inflammation can be activated in the PNS independently of the CNS due to the blood-brain barrier.
Activation of neurogenic-induced inflammation leads to several major physiologic effects. These include vasodilation of surrounding tissue, leukocyte infiltration, glial cell activation, and increased production of inflammatory mediators.81 Peripheral and central glial cells (Schwann cells, satellite glial cells, microglia, astrocytes, and oligodendrocytes) are the main cell types activated in this process. Signs and symptoms associated with neurogenic inflammation in the periphery can mimic those associated with classical inflammation: erythema, edema, warmth, and pain.
The inflammatory reactions present in the CNS and PNS are different from inflammation in other portions of the body.83 This is due to multiple factors, but a central driver for this is the unique cell types present in the CNS as opposed to other tissues. There is less permeability across the blood-brain barrier, which makes activating the complement cascade more difficult. The implication of this is that T Cells are only involved in extreme conditions and the resident innate immune cells are primarily responsible for interaction with pathogens.83 Neurogenic inflammation can also be triggered solely by increased neuronal activity (including psychological stress) and not necessarily pathogenic insults. Vascular changes associated with early CRPS have been linked to neurogenic inflammation83 as well as psychiatric disorders, such as bipolar disorder, major depressive disorder, anxiety, and cognitive deficits.82
Many of the drugs currently used for pain act to decrease neurogenic inflammation. Interestingly, opioids increase neurogenic inflammation by activating innate immune cells in the CNS.83 These inflammatory changes can also sensitize Lamina I cells to respond to non-noxious stimuli leading to allodynia.84 Vasic and Schmidt have studied hippocampal neurogenesis and have demonstrated that the inflammatory cytokines that are preferentially released in response to neurogenic inflammation can predict a patient’s proclivity to develop chronic pain and can, in contrast, predict resilience to pain.82,84 These inflammatory processes can also initiate structural and functional changes that can lead to ectopic activation of nociceptive pathways.85
Major Types of Segmental and Supraspinal Neurotransmitters
Pain is an unpleasant but vital protective function with biological and psychosocial contributory mechanisms.9,86 The amount of pain experienced is not simply a byproduct of receptor activation and signal transmissions. Instead, it is the summation of signal transmission coupled with segmental and supraspinal modulation. Within this section, we will discuss common segmental and supraspinal neurotransmitters and their role in the facilitation and modulation of pain signal transmission.
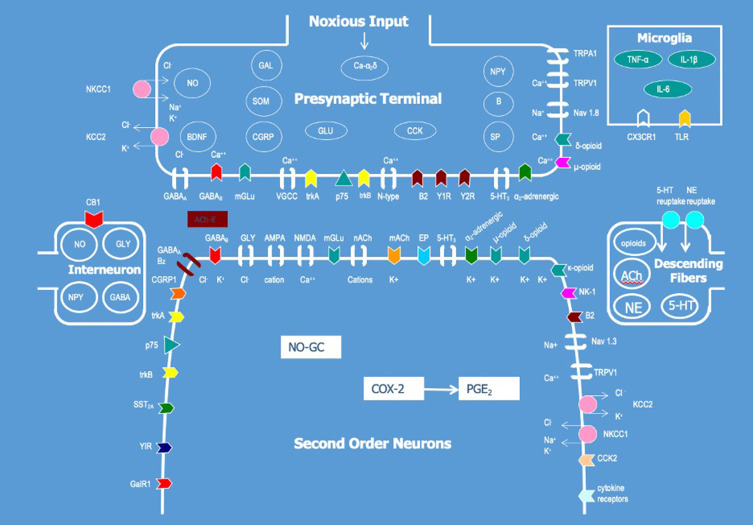
Substance P and calcitonin gene-related peptide (CGRP) are neuropeptides found within the DRG and dorsal horn involved in sensitization. Substance P is a Tachykinin87 released within the spinal cord following noxious stimuli. It acts on Neurokinin 1 and 2 receptors which facilitate sensitization by increasing the synaptic actions of excitatory amino acids.88 CGRP has two major forms, alpha and beta, and is expressed by small unmyelinated primary afferent fibers, within the DRG, and superficial layers of the spinal cord.89,90 Together, levels of substance P and CGRP are increased by neuropeptide Y, leading to excitatory effects on wide dynamic range neurons.89
Byproducts of the inflammatory cascade also play an important role in pain facilitation. Bradykinin is a peptide of the kinin family91 that facilitates pain and hyperalgesia through the activation of B1 and B2 receptors.92 This results in activation of phospholipase C, protein kinase C, modulation of transient receptor potential vanilloid 1 (TRPV1), as well as production of prostaglandins and nitric oxide.93 Eicosanoids such as Prostaglandins have been shown to cause a reduced activation threshold of tetrodotoxin-resistant sodium currents in nociceptors and raise intracellular cAMP levels facilitating excitability of sensory neurons.88 Leukotriene B4 has been linked to hyperalgesia and found in high concentrations within the joints of Rheumatoid Arthritis patients94 and in concentrations three times higher within the brains of disease groups when compared to controls.95 Cytokines, such as IL-1B and TNF-alpha, can also directly excite and sensitize nociceptive afferent fibers.88 Neurotrophins such as Nerve Growth Factor (NGF) play a role in neurogenic inflammation via both direct and indirect mechanisms.88 Indirectly, NGF is generated by, and also stimulates, mast cell degranulation leading to increased histamine and serotonin release which leads to sensitization of primary afferent fibers.88,96
Tissue damage and the resulting energy mismatch also lead to the accumulation of substances in the extracellular and intracellular milieu which can facilitate pain transmission. Accumulation of the purine nucleotide ATP can itself directly activate nociceptors.97 Additionally, through action at the P2X family of receptors, present within central terminals of primary afferent fibers and lamina V and II of the dorsal horn, ATP can indirectly lead to sensitization by facilitating the release of glutamate.98 Energy crisis also leads to the accumulation of hydrogen ions (H+) which open DRG neuron-specific acid-sensing ion channels DRAISIC/ASIC-3 facilitating sensitization.99,100
Neurotransmitters facilitate pain conduction within the central nervous system through the anterolateral and dorsal column-medial lemniscal pathways with supraspinal input from the brainstem, diencephalic, and cortical structures.88 Glutamate, along with aspartate, are the primary excitatory neurotransmitters that act on excitatory amino acid (EAA) receptors. EAA receptors are found on dorsal root ganglion cells and presynaptic terminals of primary afferent fibers.101 The inhibitory neurotransmitter GABA acts on GABA A receptors located on both unmyelinated primary afferents and DRG cells which may decrease hypersensitivity in neuropathic pain.88
Two additional molecules with both segmental and supraspinal effects are opioids and cannabinoids. Opioids act on µ receptors on peripheral terminals of afferent fibers, within the DRG, and in the central nervous system at the spinal cord, brainstem, and periaqueductal gray (PAG) resulting in both inhibition of ascending nociceptive transmission as well as activation of descending inhibition supraspinally.102 Cannabinoids act on CB1 receptors, which are present centrally and highly expressed within the DRG. Within the DRG cannabinoids decrease the release of neurotransmitters attenuating mechanical and heat hypersensitivity.102,103
Pain Receptors and Their Stimulation
Pain can be categorized into two main types: acute pain and chronic pain. Acute pain typically arises due to direct tissue damage and is often sharp and immediate.104
Acute pain can be broken down into two distinct phases. In the initial phase, the brain sends signals to alert the body to a potential threat or injury, lasting several seconds. The subsequent phase, known as the subacute phase, involves the body’s efforts to initiate protective mechanisms for tissue recovery, spanning hours or longer. The key factor distinguishing pain from nociception is the consciousness of the tissue-damaging stimulus.105 Nociception, involves specialized nerve fibers (nociceptors) that detect potential thermal, mechanical, or chemical stimuli.2 Sensory event perception denotes the transformation of these stimulus events into chemical tissue events, triggering the activation of afferent pathways.106 These afferent pathways generate sensations like pain (nociception), temperature (thermosensation), and touch (mechanoreception). Nociceptors serve as specialized primary afferent neurons responsible for transmitting noxious stimuli to the brain’s higher centers. These receptors have free nerve endings found on cell bodies in the dorsal root ganglia, with axons forming initial synapses with spinal cord cells and extending into the peripheral nervous system.107
Nociceptors are categorized into two primary classes. The first comprises medium-threshold myelinated Aδ-fibers, which convey “first” or rapid pain signals with a diameter of 1–6 μm and a velocity of 5–36 m/s.108 Aδ-fibers function as both mechanical and thermal nociceptors. The second class consists of high-threshold unmyelinated C-fibers, responsible for “second” or slow pain transmission with a diameter of 0.2–1 μm and a velocity of 0.2–1 m/s. These C-fibers specifically serve nociceptive functions.109 Further subcategorization of Aδ-fibers reveals two main classes. Type I (HTM: high-threshold mechanical nociceptors) convey first pain responses to both chemical and mechanical stimuli but possess high heat thresholds (>50). Type II Aδ-nociceptors exhibit much lower sensitivity to thermal stimulation but have notably high mechanical thresholds.106
Various forms of pain arise from different sources: 1. Physiologic pain arises from surface irritation caused by noxious stimuli on skin receptors. 2. Inflammatory pain emerges as a response to tissue injury or subsequent tissue reactions. 3. Neuropathic pain results from a primary dysfunction within the peripheral or central nervous system. 4. Dysfunctional pain stems from malfunctions within the somatosensory system itself, lacking identifiable noxious stimuli.110
The development of these stimuli arises from the response of these nerve fibers, triggering the activation of ion channels/receptors or the release of neurotransmitters.111 Inflammatory mediators are released following injury and inflammation. Consequently, these mediators interact with specific receptors in primary nociceptive neurons.112
The heightened activity in nociceptors leads to the opening of ion channels and receptors, prompting the release of neurotransmitters from central terminals within the spinal cord. This process results in central sensitization (secondary hyperalgesia and allodynia).113 Abnormal expression of receptors and ion channels during chronic pathophysiological states contributes to atypical pain signaling, resulting in persistent pain. Understanding the molecular mechanisms behind these events targets the modulation or altered functions of nociceptors due to inflammation or injury.114
Pain Transmission in the Central Nervous System
Diverse types of information are conveyed to the brain by afferent sensory nerves that consist of receptors. These receptors respond to stimuli within the skin and tissues and are activated by specific stimuli, generating electrical impulses or action potentials within the sensory nerve. Action potentials are transmitted to the nerve cell body located in the dorsal root ganglion within the spinal cord and by way of spinal cord nerves, and are subsequently transmitted through pathways that include the spinothalamic tract. A network in the brain then facilitates the transmission of these signals. From periphery, the sensory information travels along specific pathways.115 It progresses through the pain pathway’s progression and involves different types of nerve fibers with distinctive characteristics. Sensory neurons synapse in the dorsal horn of the spinal cord across specific areas known as laminae with second-order neurons and encompass nociceptive-specific (NS), wide dynamic range (WDR), and low threshold (LR) types. LR neurons only respond to innocuous stimuli whereas NS neurons react to high-threshold noxious stimuli and WDR neurons respond to sensory stimuli.116
These second-order neurons then relay their signal to the thalamus by way of the spinothalamic and spinoreticular tracts. Somatosensory data is processed by the thalamus and neurons are projected to diverse brain regions, such as the insula, anterior cingulate cortex, and the prefrontal cortex, as well as the primary and secondary somatosensory cortices. Thereupon, the intensity, location and duration are integrated leading to the perception of pain.117
Modulation of pain transmission at various points takes place across this pathway. Excitatory and inhibitory interneurons modify pain signals within the spinal cord. Sensory nerves are influenced by receptors like TrkA, TrkB, TrkC, or c-Ret receptors in the dorsal root ganglion and subsequently numerous receptors and neurotransmitters along the pain pathway modulate the pain signal.16,118
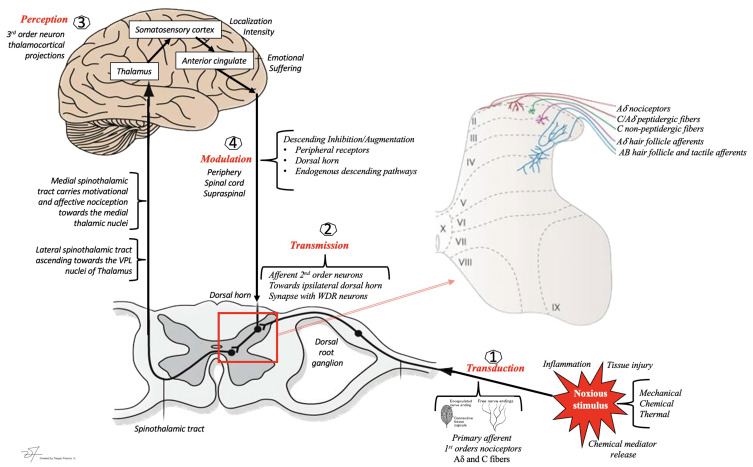
There are four major processes in the perception of a painful stimulus.
Initially, primary afferent neurons are activated by a noxious stimulus leading to transduction in peripheral axons. Secondly, in the transmission process, pain impulses travel through a two-fiber system that involves the transmission of slower sensations through C fibers and the fast, sharp sensations by A-delta fibers. Both fiber types terminate in the dorsal horn of the spinal cord. The plasticity of these dorsal horn cells allows for modulation or “gating” of pain impulses.
Second-order neurons continue the transmission to the central nervous system through both the lateral and medial spinothalamic tracts. The duration, location, and intensity of pain, projecting to the ventral posterolateral nucleus of the thalamus is conveyed by the lateral spinothalamic tract to the brain. Conversely, autonomic and unpleasant emotional perceptions of pain are conveyed to the medial thalamus by the medial spinothalamic tract.119 Signal modulation then occurs at the peripheral level altering neural activity along the pain pathway. This modulation can result in the suppression of pain. The fourth process responsible for mediating the localization, and perception of pain involves the projection from the thalamus to specific cortical regions by way of third-order neurons.2,120
Pain Pathways in the Spinal Cord and Brain Stem
Neospinothalamic Tract and Paleospinothalamic Tract
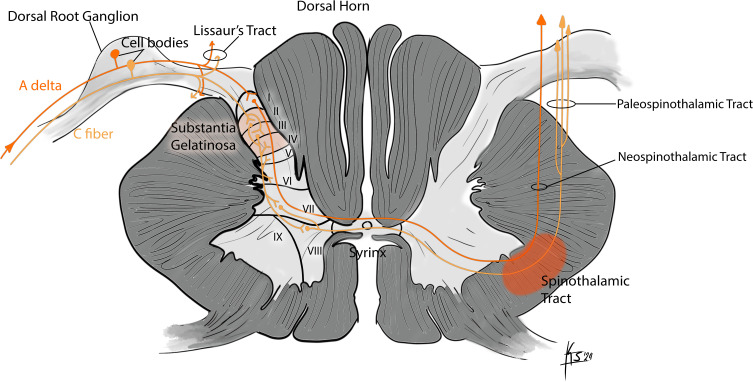
The ascending pathways that mediate pain consist of different tracts: the neospinothalamic tract and the paleospinothalamic tract. The “fast” conducting neospinothalamic pathway is involved in conveying the “sharp” pain elicited at the time tissue is damaged. The “slower” conducting paleospinothalamic pathway is involved in conveying the “dull” pain that accompanies the later inflammatory reaction in the damaged tissue as well as temperature and crude touch information.121,122 The first-order neurons are located in the dorsal root ganglion (DRG) for both of these pathways. All first-order sensory fibers, including nociceptive fibers, enter the spinal cord’s dorsal (posterior) grey horn, which is composed of several laminae (Figure 2). Each pain tract originates in different spinal cord regions and ascends to terminate in different areas in the central nervous system. This leads to different pain interpretations the body perceives in the form of acuity, character, and localization.123
Figure 2.
Rexed laminae.
There are primarily two types of fibers that transmit pain: A-delta and C-fibers. A-delta fibers are myelinated and have a larger diameter than C-fibers and thus, conduct nerve impulses faster.122 C-fibers are unmyelinated, have a smaller diameter, therefore, have a slower conduction velocity. C-fibers respond to more than one kind of stimulus (thermal, mechanical, or chemical) and thus, are “polymodal” in nature. In contrast, A-delta fibers only respond to one kind of stimulus.122
The neospinothalamic tract, also known as the direct tract, conducts fast and acute pain signals via myelinated A delta fibers. The first-order neuron, again with cell body at the dorsal root ganglion, synapses at the marginal zone, also known as the Rexed Layer I neuron, with the second-order neuron (Figure 3).121 At usually, the same level of the spinal cord, the second-order neuron crosses in the anterior white commissure to the contralateral anterolateral quadrant. This tract is referred to as the lateral spinothalamic tract. The second-order neuron synapses in the thalamus, the ventroposterolateral (VPL) and the ventroposteroinferior (VPI) nuclei. The VPL is thought to be the principal sensory relay nucleus, mainly concerned with site-specific discriminatory functions in the body.122 Neurons in the ventroposteromedial (VPM) nucleus receive nociceptive information from the face. The VPL and VPM are significant for the localization of pain. Thus, the localization of pain occurs at the level of the thalamus and not the cerebral cortex, which is responsible for assessing the quality (type) of the pain, not its location.
Figure 3.
Presynaptic and postsynaptic neurotransmitters and their receptors.
The third-order neospinothalamic neuron then synapses on the somatosensory cortex, where it is somatotopically oriented.123 Again, this pathway is responsible for the immediate awareness of a painful sensation and for awareness of the exact location of the painful stimulus.
The paleospinothalamic tract conduct a slow pain signal via unmyelinated C fibers, which is poorly localized in nature. The first-order neuron, as in the neospinothalamic tract, has its cell body at the dorsal root ganglion. This first-order neuron synapses at the substantia gelatinosa and the nucleus proprius, also known as the Rexed Layer II and III neurons, respectively. The nerve cells that furnish the paleospinothalamic tract are multi-receptive or wide dynamic range nociceptors.124
The second-order neurons make quick synaptic connections in laminae IV–VIII of the dorsal horn of the spinal cord. The second-order neurons also receive input from mechanoreceptors and thermoreceptors. Most, but not all, of their axons cross and ascend in the spinal cord primarily in the anterior region and thus called the anterior spinal thalamic tract (AST). Some of the fibers that do not cross ascend in the ipsilateral spinoreticular tract.123,124
These fibers are contained in several tracts, and these fiber tracts are collectively known as the paleospinothalamic tract. Each of them makes a synaptic connection in different locations:123,124
- Most (approximately 85%) of these second order neurons synapse in the reticular formation. The reticular formation controls the arousal and wakefulness of the central nervous system. It alerts the cerebral cortex of the perception of pain.
- From the reticular formation, the third order neuron also sends tracts to the intralaminar nuclei (discussed below).
- Some (approximately 15%) of these second order neurons synapse in non-specific nuclei of the thalamus which are called intralaminar nuclei. There are two particular nuclei thought to be involved in this tract.123,124
- Parafasiculus (PR) and Centromedian (CM) Nuclei.
- From this, third order neurons travel to several different structures including the cingulate gyrus, anterior insular cortex and somatosensory cortex.
- These structures are thought to be involved in processing the emotional components of pain. The limbic structures, in turn, project to the hypothalamus and initiate visceral responses to pain. The intralaminar nuclei also projects to the frontal cortex, which in turn projects to the limbic structures where the emotional response to pain is mediated.
Within this system, there are collateral pathways that form different tracts (spinotectal, spinomesencephalic, spinohypothalamic tracts). These tracts involve important structures including and in the periaqueductal gray (PAG), tectum, amygdala, hypothalamus. Again, these tracts facilitate different aspects of pain including pain suppression, autonomic responses, and emotional association.125
Pain Suppression System in the Brain and Spinal Cord
Brain’s Opiate System - Endorphins and Enkephalins
Pain is a complex ubiquitous and a fundamental sensory experience that alerts the body to potential threats and promotes self-preservation.126 When pain becomes chronic or overwhelming, it can lead to a myriad of physical and psychological issues and to mitigate the impact of pain, the human body employs a highly evolved pain suppression system that operates in the brain and spinal cord.127 The opiate system consists of a network of receptors, endogenous opioids peptides, including endorphins and enkephalins, playing a key role in pain modulation.103,128
There are three primary types of opioid receptors in the human body: mu (μ), delta (δ), and kappa (κ) receptors. These receptors are distributed throughout the central nervous system, including the brain and spinal cord, as well as the peripheral nervous system.129–131 Each type of receptor has distinct functions in pain regulation and physiological processes. The action of mu, delta, and kappa receptors can be summarized in Table 1.
Table 1.
Receptors and Their Action
| Receptors | Action | Peptides |
|---|---|---|
| Mu | Inhibits nociception | Endorphins, Enkephalins |
| Delta | Pain modulation | Endorphins, Enkephalins |
| Kappa | Analgesia | Dynorphins |
Neuropeptides in the nervous system play crucial roles in modulating neuronal activity (Tables 2 and 3). Inhibitory neuropeptides, such as somatostatin and enkephalins, dampen neural firing by hyperpolarizing neurons or reducing the release of excitatory neurotransmitters. These neuropeptides help regulate pain perception, anxiety, and overall neural excitability. On the other hand, excitatory neuropeptides, like substance P and dynorphins, stimulate neural activity by depolarizing neurons and increasing neurotransmitter release.89,132 They are involved in processes such as pain transmission, stress responses, and mood regulation. The balance between inhibitory and excitatory neuropeptides is essential for maintaining proper neural function and emotional well-being within the nervous system. The complexity of the neural pathways can be seen in Figure 3.132
Table 2.
Excitatory Neurotransmitters and Their Receptors
| Excitatory Neurotransmitters | Receptor |
|---|---|
| Glutamate | NMDA, AMPA, kainate |
| Aspartate | NMDA, AMPA, kainate |
| Substance P | Neurokinin 1 and 2 |
| neurokinin | Neurokinin 1 and 2 |
Table 3.
Inhibitory Neurotransmitters and Their Receptors
| Inhibitory Neurotransmitters | Receptor |
|---|---|
| Glycine | CLSS (chloride-linked strychnine-sensitive), SI (strychnine insensitive) |
| GABA | GABA a |
| Norepinephrine | Alpha 1, alpha 2 |
| Adenosine | Adenosine receptor, A1 and A2 |
| Acetylcholine | Muscarinic, nicotinic |
Endorphins and enkephalins are two families of endogenous opioid peptides that bind to opioid receptors in the brain and spinal cord.128 These peptides are produced within the body and act as natural painkillers. Endorphins are produced in response to stress and pain, and they induce a sense of euphoria and well-being. Beta-endorphins are one of the most well-known endorphins, and they bind primarily to mu receptors, contributing to pain relief. Enkephalins are another group of endogenous opioids that play a significant role in pain modulation. They bind to both delta and mu receptors, providing analgesic effects.
When pain signals are transmitted from peripheral nerves to the spinal cord and brain, endorphins and enkephalins can inhibit the release of neurotransmitters like substance P.133 This inhibition reduces the transmission of pain signals, effectively dampening the perception of pain.
Endorphins and enkephalins can alter the perception of pain by increasing the pain threshold. This means that a person may tolerate a painful stimulus better when the opiate system is activated, resulting in reduced pain perception. Activation of the opiate system can also influence emotional responses to pain. Euphoria and a sense of well-being produced by endorphins can help individuals cope with pain more effectively, both physically and psychologically.
Opioid medications, such as morphine and codeine, work by mimicking the actions of endogenous opioids, and they are used to manage moderate-to-severe pain. The opiate system, with its endogenous opioid peptides like endorphins and enkephalins, plays a pivotal role in regulating pain perception within the brain and spinal cord. Understanding the mechanisms by which these endogenous opioids modulate pain provides valuable insights into both physiological processes and potential therapeutic interventions for pain management.
Pain Processes
Pain is an intricate sensorial experience that involves biological, cognitive and psychosocial factors134,135 Four essential steps encompass the transformation of a noxious stimulus into the awareness of pain: transduction, transmission, modulation and perception at higher cortical centers.136
Transduction
Transduction of pain is a complex process that relates to the conversion of an evoked electrochemical action potential from a mechanical, thermal or chemical noxious stimulus that activates primary afferent first-order neurons distributed throughout the body (skin, viscera, muscles, joints, etc). Inflammatory mediators such as bradykinin, serotonin, prostaglandins, leukotrienes, and cytokines are released from damage tissues, which thereby stimulate these specialized peripheral nociceptors, such as Aδ-fibers and C-fibers. High-threshold receptors respond to mechanical deformation, while polymodal nociceptors respond to a variety of inflammatory chemicals.108,137–139 Their properties are summarized in Table 4.
Table 4.
Characteristics of Primary Afferent Fibers
| AB fibers | Aδ-fibers | C fibers | |
|---|---|---|---|
| Diameter | Large | Small | Smallest |
| Conduction velocity | Very Fast | Fast | Slow |
| Myelination | Heavily myelinated | Lightly myelin | Unmyelinated |
| Stimulus Sensation Function |
Non-noxious Light touch Proprioception Mechanoreceptor |
Noxious, rapid Mechanical and thermal Acute, sharp pain Nociception |
Noxious, slow Polymodal (chemical, mechanical, thermal) Dull, aching pain Nociception |
Transmission
Transmission is the process of conveying the nociceptive message from the peripheral nervous system (PNS) to the central nervous system (CNS) by the primary afferent nociceptive neurons (Aδ-fibers and C-fibers). These fibers terminate at the CNS, more specifically at the lamina I and V and I–II of the spinal cord, respectively. Their cell body is located at the dorsal root ganglion (DRG), which sends one branch to the spinal cord and one back to the periphery.3,108,136–139
Peripheral Nervous System
The PNS can be divided into somatic nervous system and visceral nervous system. Each division has a motor and sensory component, including the autonomic nervous system (sympathetic, parasympathetic, enteric). The somatic nervous system includes the sensory system, which consists of cranial nerves (except the optic nerve) and spinal nerves (cervical and lumbosacral plexus). The somatosensory nervous system consists of peripheral sensory receptors and the primary and secondary somatosensory cortex.140,141
Pain Pathways in the Central Nervous System
The pathway of pain begins in the periphery with activations of nociceptors where transduction occurs, followed by transmission by primary afferent neurons to synapse in the dorsal horn of the spinal cord to enter the CNS.108
Nociception and Peripheral Sensitization
Nociception differs from pain. Nociception is the physiological activation of neural pathways from peripheral noxious input, which results in a behavioral, withdrawal or escape response (Lee 2020). Peripheral sensitization is a decrease in threshold and an increase in responsiveness of these peripheral nociceptors, often spontaneously, that occurs after tissue damage and inflammation, resultant from early posttranslational changes with phosphorylation of ion channels (G-protein couple receptors or tyrosine kinase receptors) and altered gene expression leading to primary hyperalgesia.42,142
Dorsal Horn Neurons and Central Sensitization
In contrast to peripheral sensitization, central sensitization is a consequence of changes within the CNS that leads to alterations of sensory input interpretation, which ultimately results in enhanced synaptic transfer. This is characterized by heterosynaptic potentiation, membrane hyperexcitability in microglia, astrocytes, gene transcription, and wind-up. Wind-up phenomenon is an increase in action potential output from dorsal horn neurons during a low-frequency firing of C-fibers, which leads to higher calcium levels and the release of glutamate, substance P and calcitonin gene related peptide (CGRP) resulting in an increased postsynaptic depolarization of dorsal horn neurons.42,142 The dorsal horn is comprised of cell bodies (sensory nuclei) of second-order neurons and their synapses with pseudounipolar first-order sensory neurons located at all spinal levels. Complex interactions occur at the dorsal horn with wide dynamic range neurons (WDR), excitatory and inhibitory interneurons releasing glycine and gamma-aminobutyric acid (GABA) to modulate nociceptive transmission, activate ascending and descending pain pathways and a spinal “gate control” system.136,138,139
The Ascending Tracts—Sensory-Discriminative Pathways and Affective Pathways
From the dorsal horn, these neurons synapse with interneurons and WDR neurons to ascend via second-order neurons contralaterally at the spinothalamic tract (neospinothalamic), medial spinothalamic (paleospinothalamic) tract, and spinoreticular tract. The characteristics of these ascending pathways are summarized in Table 5.136,139
Table 5.
Characteristics of Ascending Pain Pathways
| Lateral Spinothalamic Tract | Medial Spinothalamic Tract | Spinoreticular Tract | |
|---|---|---|---|
| Origin | Neurons in lamina I and V | Neurons in lamina II and IV, VIII | Deep gray matter and lamina V |
| Second Order Neurons | Anterior white commissure | Anterior white commissure | Anterior lamina VII and VIII and posterior gray horns lamina V |
| Third Order Neurons | Ventral posterior lateral (VPL) thalamic nucleus, ventral posterior inferior (VPI) nucleus and to post-central gyrus | Medial thalamic nuclei, periaqueductal gray matter (PAG), somatosensory cortex and limbic centers | Thalamus and hypothalamus via brainstem reticular formation and limbic centers |
| Structure | Neospinothalamic tract with somatotopic organization (caudal elements lateral, rostral elements medial) | Paleospinothalamic tract, little somatotopic organization | Phylogenetically ancient with little somatotopic organization |
| Function | Sensory discriminative (localization) aspect of pain | Autonomic, affective, motivational components of pain | Perception of diffuse, emotionally disturbing pain Alertness and arousal of pain |
The Cortical Pain Matrix and the Psychological Components of Pain
Complex activation of multiple cortical regions comprise the pain matrix responsible for the perception and awareness of painful stimuli, including primary (S1) and secondary somatosensory cortices (S2), limbic regions (cingulate, insula, amygdala), brainstem, hippocampus, parietal operculum, cerebellum, and the orbitofrontal, anterolateral and prefrontal cortices.143,144 These can be categorized into three different levels, primary, secondary and third cortical matrices (Table 6). The first and secondary matrices interact with each other, and the third cortical matrix receives and integrates the information from the foregoing two cortices and triggers a behavioral response.144
Table 6.
Characteristics of Pain Cortical Matrices
| Primary Cortical Pain Matrix | Secondary Cortical Pain Matrix | Third Cortical Pain Matrix | |
|---|---|---|---|
| Structures | Primary somatosensory Secondary somatosensory Parietal operculum Posterior insula |
Anterior cingulate cortex Anterior insula Amygdala Hippocampus |
Frontal cortex (orbitofrontal, anterolateral, prefrontal) Medial cingulate Posterior cingulate |
| Function | Pain perception Pain localization Pain quality Pain Intensity |
Affective experience Emotional experience Negative emotions Distress Unpleasantness |
Cognitive meaning Behavioral response |
Modulation of Pain—Gate Control, the Descending Modulatory System, and Neuromodulators
Neuronal circuits that modulate pain include descending systems from higher centers, spinal cord gate control mechanisms and endogenous opioids.138
Gate Control
The gate control theory of pain was proposed by Melzack and Wall in 1965 to describe a process of inhibitory pain modulation at the spinal cord level by activating Aβ fibers with tactile, non-noxious stimuli towards inhibitory interneurons in the dorsal horn (lamina II), which once activated lead to inhibition of pain signals transmitted via C fibers.7
The Descending Modulatory System
Once a pain signal reaches the cortical pain matrix, it triggers a reflexive-descending modulatory pathway from the anterior cingulate cortex, amygdala and hippocampus to brainstem nuclei in the PAG area of the midbrain to allow individuals to function and to respond to pain stimulus (Figure 4). The PAG receives pain stimulus from the spinal mesencephalic tract and relays to the rostral ventral medulla, which thereby activate the endogenous opioid system and project to the dorsal horn to modulate pain transmission. These pathways are monoaminergic and utilize noradrenaline and serotonin neurotransmitters from the nucleus raphe magnus.138 There are three types of G-protein couple opioid receptors within the CNS (mu, delta, and kappa) that upon activation results in neurotransmitter inhibition, cell hyperpolarization, and reduction of cell excitability.42,142 Endogenous opioids, such as Β-endorphins predominately binds to mu opioid receptors, Dynorphins predominately bind to kappa opioid receptors and Enkephalins predominately bind to delta opioid receptors.138
Figure 4.
Complex pain pathways: stimulation of nociceptive nerve endings from joints, muscles/tendons, and skin are transmitted from the periphery to the spinal cord to the cortex in a complex pathway involving transduction, transmission, and modulation of the pain signal.
Sensory versus Affective Aspects of Pain
The experience of pain involves a complex interaction between the PNS, CNS and higher-cortical centers. The sensory discriminative aspect of pain refers to intensity, spatial and temporal characteristics, and quality of pain, while the affective-motivational dimension captures how unpleasant the pain is and the behavior to adapt to it.145 The sensory aspect concerns to somatotopic localization, detection and identification of the noxious stimulus, which can be described with a sensory quality, such as burning or sharp pain. In contrast, the affective or unpleasantness aspect of pain correlates with the aversive drive to terminate the noxious stimulus, often described by terms that are not specifically tied to a sensory experience, such as excruciating, unbearable, nagging, or uncomfortable.3 The affective experience of pain is regulated by projections between higher-cortical centers, such as the primary somatosensory cortex that transmit sensory information (sensory aspect) to the anterior cingulate cortex (Singh 2020). The difference between the sensory and affective aspects of pain can be illustrated further by distinguishing between pain threshold (sensory component) and pain tolerance (affective component), which varies widely among individuals. Pain tolerance is a complex interaction that may be modified by previous experiences, personality traits, gender and psychosocial factors.3
In chronic pain states, studies have found that there is a higher firing rate of the primary somatosensory cortex leading to an environment of enhanced maladaptive synaptic transmission in pyramidal neurons to the anterior cingulate cortex, resulting in augmented sensory hypersensitivity and increase aversion of pain.146 The primary, secondary and third pain cortical matrices are not activated separately but are functionally connected and contribute in a combined fashion to pain processing. As such, the intensity and degree of pain experience can result in changes in emotional and motivational behavioral, such as catastrophizing, and vice versa. Given this multinetwork activation in the CNS in such pain states, it is vital to have a foundation knowledge of pain pathways, mechanisms of neuromodulation and how the pathophysiology of chronic pain affects multiple elements of human behavior, including sensory, physical, emotional, cognitive, reward-related, and psychosocial elements.147
Physiological Processes That Enhance Pain and Lead to Chronicity
Sensitization and Hyperactivity of the Sympathetic Nervous System
Maladaptive responses can lead to pain chronicity that extends painful symptoms beyond the healing period. Several physiological processes have the ability to enhance pain and lead to chronicity. The sympathetic nervous system plays an important role in the body’s response to pain and stress and may be dysregulated in chronic pain.148,149 Dysregulation of the sympathetic nervous system contributes to many chronic pain states.150 When this occurs, there are increased levels of norepinephrine, which leads to vasoconstriction, reduced tissue perfusion, and heightened pain sensitivity. There is also evidence that sympathetic hyperactivity leads to neuroinflammation and central sensitization, further perpetuating the pain experience.151
Muscle Contraction
Muscle contraction and the resulting myofascial tension and pain are common in many chronic pain states. Prolonged muscle contraction may lead to ischemic changes in the tissue, accumulation of metabolic byproducts, and sensitization of nociceptors.152 Muscle contraction and tension may lead to the development of myofascial trigger points, which will lead to localized areas of hypersensitivity with pain that may radiate.
Self-Sustaining Painful Processes
Chronic pain is notoriously characterized by a self-sustaining painful process that perpetuates the pain independent of the inciting event. The mechanism by which this occurs is a complex interplay of neurobiological and psychosocial factors, which ultimately lead to persistent pain, pain amplification, and maintenance of pain. This may be via neuroplastic changes in the central nervous system that could comprise altered synaptic transmission, maladaptive neuroplasticity, and neuronal sensitization.153 These processes make chronic pain especially difficult to manage.
Acute versus Chronic Pain
Acute pain is often proportional to the inciting event or injury. This serves a protective and useful function to allow for tissue healing. Acute pain typically resolves with the healing process. Unlike acute pain, chronic pain involves maladaptive changes that sustain the painful experience. Chronic pain may persist long after the inciting event has resolved, and the severity of chronic pain is often out of proportion to the initial injury.
Peripheral Nerve Stimulation
Introduction
The earliest use of direct electric current to stimulate a peripheral nerve was recorded in the mid-1800’s.154 Advances in PNS were rapidly accelerated in the 1980’s with the advent of readily available cylindrical leads, paddle leads, and implantable pulse generators.155 Weiner and Reed later demonstrated a percutaneous technique for treating occipital neuralgia.156 Subsequent technological advancements and clinical research has advanced the application of PNS to a variety of nerve targets for both pain as well non-pain applications.157,158
Mechanism of Action
The mechanism of action of PNS is likely a combination of central and peripheral mechanisms.159 Proposed mechanisms include gate control theory,7 local changes through regulation of inflammatory mediators, neurotransmitters, and endorphins,160 potential failure of excitation with repeated stimulation of A and C fibers,161 and peripheral reconditioning of the central nervous system.162
The Peripheral Pathway Mechanism
PNS has been shown to have local effects including downregulation of endorphins, neurotransmitters, inflammatory mediators and blood flow.154,163 Decreased ectopic discharge has been confirmed by electrophysiologic studies, decreasing efferent transmission of nociception.164 Tetanic stimulation of nerves results in decreased excitability and conduction velocity, and targeted research on radial and saphenous nerves has demonstrated excitation failure in A and C fibers.154,161 Nerve regeneration has also been demonstrated in rat models with PNS, seemingly dependent on stimulation parameters.165
The Central Pathway Mechanism
In 1965, Melzack and Wall proposed that applying non-painful stimulation to A beta fibers activates inhibitory interneurons and inhibits nociceptive A delta and C fiber conduction as well as discharge in the dorsal horn and thereby the central cortex.7,154 PNS may result in modulation of higher CNS centers.166–168 Induced changes in serotonin, dopamine, substance P, CGRP have all been posited to play a role.169–171 Inhibition of WDR neurons, a mechanism also implicated in SCS stimulation, has been demonstrated with PNS.168 Further supporting a central mechanism, tibial nerve stimulation has been shown to increase inhibitory activity at the bladder.172,173
Deer et al described a theory of peripherally induced central reconditioning with PNS whereby selective stimulation of large vs small diameter nerve fibers peripherally results in pain relief in the short term via spinal mechanisms, including gate-control theory.162 However, they posit that long-term sustained relief is mediated by a change in the somatotopic representation map in the primary somatosensory cortex where overrepresented painful areas may be decreased in size via targeted, robust, peripheral stimulation of nerves in a way that cannot be easily replicated by conventional SCS.174–176
Applications of Peripheral Nerve Stimulation
The use of PNS has been applied to a variety of nerve targets and treatment indications, including shoulder, knee, lower extremity, trunk, pelvic, low back pain, occipital nerves, and CRPS, as is well described in the literature.177–179
Overall evidence for PNS was rated as Level I across 14 RCTs.177 The ASPN PNS Best Practice Guidelines graded evidence to support head and neck, upper extremities, low back/trunk, and lower extremities as Level I.159 Real world applications of PNS include both short-term treatment modalities as well as permanent implantation, with both options supported by strong evidence.178–180
Management of Acute Pain
PNS has been studied in the treatment of acute pain, primarily post-operative pain.154 One of the earliest studies employed leads placed at the femoral or sciatic nerve in patients who underwent total knee arthroplasty and demonstrated pain reduction of 63% within 2 hours.181 Subsequent studies have examined the utility of short-term PNS treatment for patients following foot surgery and shoulder surgery.182,183 A recent study found the use of post-operative PNS results in reduced opioid requirements and pain scores during the 7 days following surgery.184 The safety profile for the use of PNS in acute pain is excellent with fewer than one infection reported per 32,000 indwelling lead days which is markedly lower than rates reported for perineural catheters.185
Management of Chronic Pain
There is a broad array of literature supporting treatment for a variety of chronic pain conditions with PNS. The pain targets that have been most extensively studied include shoulder pain, knee pain, and low back pain.178,180,186 Medial branch nerve stimulation for the treatment of low back pain has been studied extensively with short-term treatment resulting in greater than 1 year of sustained relief and long-term treatment periods showing benefit even at 3 year follow-up.178,187 Multiple studies have shown reduction in pain intensity and headache days with occipital nerve and sphenopalatine ganglion stimulation.177,188 Hardware improvements, evolving research, and personal success has led to the application of PNS to a seemingly endless list of nerve targets.
Spinal Cord Stimulation
Spinal Cord Stimulation Parameters
The most basic parameters involved in adjusting the delivery of SCS charge are amplitude, pulse width, and frequency.189 The amplitude denotes the amount of current delivered in a pulse and is usually measured in milliamperes (mA).189 A higher amplitude correlates with greater recruitment of nerve fibers, higher perceived intensity of stimulation, and higher perceived paresthesia sensation.189 After a pulse is delivered, it is subsequently followed by a recharge pulse, in which an equal flow of current is delivered in the opposite direction.189 The recharge pulse can be either active or passive.189 The pulse width is the specific duration of time of current delivery, usually measured in microseconds (µs).190 An increase in pulse width correlates with a greater recruitment of nerve fibers.190 Further, an increase in pulse width may preferentially recruit smaller diameter nerve fibers and may result in broader paresthesia coverage.190 Frequency denotes the number of pulses that are delivered per unit of time, usually measured in Hertz (number per second). The frequency may determine how often a neuron may fire after a stimulus. Conventional SCS typically delivers SCS charge in the range of 40–100 hz, which leads to paresthesia sensation. Many central neurons fire at rates less than 200 hz, meaning that these nerves can entrain electrical stimulation that is at 200 hz or under.191 In summary, the charge delivery per pulse, frequency, and recharge properties play a major role in the response of neurons to neuromodulation. When adjusting basic SCS parameters, each variable is related and should be considered collectively. For instance, an increase in frequency would imply a decrease in pulse width. As another illustrative example, a high-amplitude high-frequency paradigm may result in a complete reversible nerve block, while a low-amplitude high-frequency paradigm would have a different physiological mechanism.192
Overview of Paresthesia-Based/Tonic Spinal Cord Stimulation
Tonic stimulation is typically programmed to have a pulse width between 100–500 µs, frequency between 30–100 hz, and an amplitude that produces paresthesias at a comfortable level.193 The gate-control theory has conventionally been used to explain the mechanism of analgesia from paresthesia-based spinal cord stimulation, in which activation of large-diameter A-beta fibers inhibits A-delta and C fibers via interneurons.7,194 However, recent literature suggests that the mechanism of SCS is more complex. In the dorsal column, SCS may upregulate inhibitory neurotransmitters (eg gamma-aminobutyric acid [GABA]), downregulate excitatory neurotransmitters (eg glutamate, substance P, bradykinin, calcium gene-related peptide), modulate glial cell conduction, and attenuate the neuroimmune response.189,195 In the dorsal horn, SCS may modulate wide-dynamic-range neuron excitability. Further, supraspinal mechanisms may include orthodromic activation of the dorsal column via descending inhibitory pathways from the brainstem, which is mediated via serotonin, norepinephrine, and adenosine.196 The complexity of these mechanisms may become even more sophisticated when considering distinct pain syndromes and their response to distinct modalities of SCS.
10 kHz Spinal Cord Stimulation
Variable frequencies of SCS induce unique physiological mechanisms. At low-frequency stimulation (eg 4–60 hz), release of endogenous opioids and binding of neurotransmitters to I-opioid receptors occur.197 At frequencies typically used in tonic stimulation (eg 60 hz), activation of GABAergic neurons in the dorsal horn, activation of the d-opioid system, and modulation of the rostroventromedial medulla and locus coeruleus may occur.197 When SCS frequency reaches 500 hz, an increase in peripheral blood flow may occur.198 At much higher frequencies of SCS (200–10,000 hz) that are significantly higher than the firing rate of central neurons, there is inadequate time for sensory information processing. SCS at 10 kHz utilizes low intensity with a short-duration pulse width, delivering paresthesia-free stimulation and leading to direct neural inhibition via dorsal horn inhibitory neurons.199,200 Further, there is a reduction in the activity of wide-dynamic range neurons. Preclinical studies in mice comparing 1-kHz, 5-kHz, and 10-kHz SCS revealed that 10-kHz SCS had greater selectivity for dorsal horn neurons.199 These differences in mechanisms may also potentially explain differences in clinical studies comparing paresthesia-based waveforms with 10-kHz waveform.201
Very High Frequency Stimulation (> 10 kHz)
There are limited data on very-high frequency stimulation, which involve frequencies over 10 kHz up to 500 kHz. Unique mechanisms of action with very-high frequency stimulation include conduction block of axons, desynchronization of activity in axons, and modulation of glial-neuronal interactions.202,203 However, conflicting evidence has been demonstrated in other models that very-high frequency stimulation is unlikely to block or desynchronize axonal transmission. For instance, in a preclinical rat study, few axons generated action potentials with stimulation frequencies ranging from 1 to 20 kHz and stimulation amplitudes below 50% of the motor threshold.203 Further, conduction block occurred rarely. The mechanisms and potential dosing of very-high frequency stimulation such as 500 kHz is currently being evaluated, in both animal and human models.
Burst Spinal Cord Stimulation with Passive Recharge
The proprietary burst SCS, as described by DeRidder, with passive recharge is defined by series of five 1000-µs pulses delivered at a frequency of 500 hz and an interburst frequency of 40 Hz.204 Charge balancing occurs passively after five 1000-µs pulses.204 The mechanism of analgesia in burst SCS with passive recharge includes GABAergic interneuron activation in the dorsal horn, which can be reversed with administration of intrathecal GABA-A and GABA-B antagonists.205 While tonic SCS is known to stimulate the lateral spinothalamic tract, studies suggest that burst SCS stimulates both the medial and lateral spinothalamic tracts. The medial spinothalamic tract conveys fibers that transmit the emotional and affective components of pain.206 Furthermore, unique supraspinal mechanisms are noted with burst SCS. For instance, preclinical studies highlight a delayed wash-in effect from burst SCS may activate supraspinal areas involved in emotion and motivation.206,207 Burst SCS may also modulate the synaptic connectivity in the thalamic-anterior cingulate pathway, which is involved in the transition and maintenance of acute to chronic pain as well as pain-induced anxiety.205
Closed Loop Spinal Cord Stimulation
Most forms of SCS utilize open-loop systems. After patients provide feedback on stimulation-induced paresthesia and pain relief, reprogramming of SCS parameters is manually performed and then these settings are typically not readjusted unless patients present for another reprogramming clinical visit. In closed-loop SCS using evoked-compound action potentials (ECAPs), the system records ECAPs in real-time, which reflect the summation of individual action potentials from somatosensory A-β neurons of the dorsal column.208 This is important because clinical efficacy and degree of therapeutic stimulation that is delivered to the spinal cord may vary based on changes in body position. The spinal cord can move approximately 2–3 mm in the anterior-posterior direction and may lead to either overstimulation or understimulation. Factors such as body position, respiration, and heartbeat may impact the position of the spinal cord. In a closed-loop system monitoring ECAPs, the amplitude of the ECAP correlates with SCS amplitude and paresthesia coverage. The system is then able to adjust the SCS amplitude to produce a reference ECAP amplitude that the patient reports as comfortable and optimal. Although the mechanism explaining the superiority of closed-loop SCS over conventional SCS remains unclear, several hypotheses have been proposed. One hypothesis is that stimuli above the ECAP activation threshold and the greater frequency of surpassing that threshold to provide sustained and consistent neural recruitment in closed-loop SCS may provide superior analgesia.209,210
Differential-Target Multiplexed (DTM) Spinal Cord Stimulation
Glial cells, which consist of Schwann cells, oligodendrocytes, astrocytes, microglia, and ependymal cells, comprise the majority of neurons in the central nervous system and may be implicated in chronic pain states.211 For instance, ablation of oligodendrocytes was associated with worsening neuropathic pain in preclinical models.212 A proprietary waveform, known as differential-target multiplexed (DTM) involves the delivery of multiple waveforms that are charge-balanced and comprise of variable frequency and pulse width. Preclinical studies highlight that DTM SCS has the potential to promote greater modulation and diminish pain-related behaviors compared to conventional SCS paradigms.211 For instance, a higher expression of modified glial-specific gene was observed in rodent models treated with DTM SCS. In another animal model study of neuropathic pain, DTM SCS modulated protein expression that reversed dysregulation of ion transport.213 DTM SCS decreased proteins related to calcium ion transport, upregulated presynaptic proteins involved in GABA vesicle formation, regulated postsynaptic chloride ion inhibition, and downregulated potassium regulatory proteins involved in neuronal depolarization.213
Surround Inhibition Spinal Cord Stimulation
There are concentrated populations of inhibitory A-β neurons in the periphery surrounding central wide-dynamic range neurons that transmit nociceptive pain.214 By preferentially stimulating these surrounding inhibitory A-β neurons using low-frequency waveforms (surround inhibition SCS), conduction blockade of nociceptive pain is achieved in wide-dynamic range neurons.214 In surround inhibition SCS, use of paresthesia-independent and low-frequency waveform under 150 hz is utilized to selectively stimulate these inhibitory A-β neurons,215 leading to a faster onset of analgesia (seconds to minutes) and energy conservation compared to tonic SCS.216 Pre-clinical studies with computational models and in vivo experiments in rats were conducted to explore the mechanisms of surround inhibition SCS.215 The response of modeled and recorded dorsal column axons revealed irregular spikes after surround inhibition SCS, with corresponding rapid inhibition of dorsal horn neurons. Further, intrathecal administration of bicuculline, a GABA-A antagonist, impacted the neural response to surround inhibition SCS which implies that analgesia from this waveform paradigm is also dependent on GABAergic mechanisms.215
Multiphase Spinal Cord Stimulation
Due to the novelty of multiphase SCS with ongoing clinical trials, there are limited data on its mechanism of action. Most SCS modalities deliver a single therapeutic phase from the implanted electrodes. When accounting for charge balancing to prevent net direct current in single-phase SCS systems, an interruption in therapy is necessary. In multiphase SCS, there is an integrated circuit where therapy is delivered via multiple electrodes in a continuous, spatially, and temporally distributed pattern.217 By delivering current in this manner, an interruption to account for charge balancing is unnecessary and therapy can be delivered continuously. Further, multiphase SCS can deliver stimulation over a broader area of the dorsal column, with computational models estimating two to five times greater area in the rostro-caudal direction compared to most SCS modalities.217 Despite a larger area of the spinal cord being targeted in multiphase SCS, an estimated 30–60% less power is needed.
Dorsal Root Ganglion Stimulation
Spinal cord stimulation (SCS) is a minimally invasive, safe treatment that helps improve function and decrease pain,218 but comes with several limitations. One notable limitation is localization, due to lack of dermatomal or peripheral targets since the electrodes are placed into the epidural space. SCS is effective for central neuropathic processes but less so in treatment of focal and peripheral lesions causing pain in the trunk, groin, knee, and foot.219
On the other hand, dorsal root ganglion (DRG) stimulation allows for more specific targeting of the DRG that innervates the targeted painful region when compared to traditional dorsal column SCS.220 DRG stimulation (DRG-S) was first explored in 1991 in ex vivo animal models to treat pain and inflammation.221 Since then, this relatively new neuromodulation therapy has emerged with promising potential in treating chronic neuropathic pain, as suggested by the results of the landmark ACCURATE trial in 20165 and recommendations by the Neuromodulation Appropriateness Consensus Committee (NACC) in 2018.219
The DRG acts as more than a transmitter of the bulk of sensory information from the periphery to the central nervous system.219 It actively regulates afferent nociceptive signaling to the anterolateral system.222 The DRG neurons have sensory cell bodies and pseudo-unipolar morphology with T-junctions, which are axonal bifurcations.220,223 These neurons may inhibit action potentials propagating from a nociceptor to the dorsal root entry zone, contribute to the transmission of electrical signals, or act as a low-pass filter to electrical impulses from the periphery.224 They consist of somatic fibers, which originate from a consistent region corresponding to their dermatome, and sympathetic fibers, which contain information from outside the dermatome. This mix of somatic and sympathetic afferents implies each DRG contains fibers that derive from a broader region beyond the boundaries of a single dermatome.219
The DRG plays a crucial role in the pathogenesis of neuropathic pain. In response to peripheral nerve injury, the DRG becomes inflamed, inducing a hyperexcitable state225 with ectopic firing of the cell bodies226 that impairs its filtering ability at the T-junction. The surrounding glial cells, cytokines, neurotrophic factors, ion channels, and neurotransmitters contribute to pathologic changes in the DRG that perpetuate and amplify neuropathic pain, which clinically manifest as central sensitization and allodynia.227 Changes in the electrophysiological membrane, integral membrane proteins, and genetic changes are implicated in this process.228
DRG-S is thought to achieve analgesia through normalization of the hyperexcitable state of DRG neurons in neuropathic pain.229 Through a downstream effect, DRG-S not only stabilizes peripheral nociceptors but also sensitized wide-dynamic-range neurons in the dorsal horn and the potential modulation of supraspinal brain areas.230,231 Moreover, crossover fibers in the sympathetic chain and sacral plexuses are hypothesized to allow the electrical stimulation of one DRG to stabilize neighboring or contralateral segments.232 The result is pain relief in unstimulated regions that correspond to neighboring DRGs, in addition to the stimulated region that correspond to the targeted DRG.229
Sacral Dorsal Root Ganglion Stimulation
Unlike thoracic or lumbar DRG, the sacral DRG is mostly found within the spinal canal, with occasional presence in the foraminal region. None has been identified outside the foramina.233 DRG-S in the sacrum offers focal, often paresthesia-free stimulation to a painful area innervated by S1, S2, or S3 spinal nerves.220 It is worthy to note that DRG-S induces paresthesias but does not rely on them.234 While DRG-S has shown to be more effective for CRPS than SCS, it provides less paresthesia coverage in painful areas.235 Over time, more than one-third of patients become paresthesia-free without compromise in pain control.236 The DRG lies outside the blood-brain barrier enveloped by a thin layer of cerebrospinal fluid, which enables subthreshold stimulation that lead to reduction in the sensation of paresthesia.237
Sacral DRG-S may treat chronic pain in the back, foot, and pelvis. Of these, pelvic pain is often most difficult to treat due to the presence of lumbar and sacral innervations in the viscera posing challenges in simultaneously addressing all pain areas. For a patient experiencing pain in the bladder, inguinal region, and perineum, simultaneous stimulation is required across the distributions of the ilioinguinal, hypogastric, genitofemoral, pudendal, and inferior rectal nerves. This corresponds to the T12, L1, L2 as well as S2 and S3 dermatomal distributions.238 It is recommended to adhere to strict selection criteria when treating for pelvic pain. This involves identifying the mechanism of injury (whether surgical or trauma-related) and associated pathology, while also distinguishing between visceral and somatic types of pain.219
Currently, the FDA-approved sacral levels for DRG-S are S1 and S2. However, it is within standard of care to implant S3 if indicated.239 DRG-S is recommended as an effective therapy in CRPS type I and II of foot and ankle, where S1 is one of the most commonly placed DRG levels. It is also recommended in patients with chronic postoperative surgical pain of the foot and ankle on a case-by-case basis. It may be considered in phantom lower limb pain/post-amputee syndrome involving S1.219 DRG-S involving sacral region has been also studied in chronic pelvic pain, failed back surgery syndrome (FBSS) involving S1, pudendal neuralgia including testicular pain the scrotum (S2 and S3), and nerve injuries (eg radiculopathy, peripheral neuropathy) associated with S1 and S2 distribution.240 It is hypothesized that there is crosstalk between adjuvant DRGs, thereby placement of a lead at one level may lead to analgesia at adjacent levels.226 One case series reported the outcome of S1 DRG-S in five patients diagnosed with CRPS of the foot from prior lumbar spine surgery. All patients experienced substantial pain relief of the entire foot with single S1 DRG lead placement as well as improved quality of life at 6 months follow-up.241
Lumbar Dorsal Root Ganglion Stimulation
Lumbar DRGs are found in close proximity to the foraminal spaces where the nerves exit. On radiologic imaging, they are identified at the caudal aspect of the neuroforamen on AP view and posterior to the vertebral body on lateral view.233 According to one study, the L1-L3 DRGs were either extraforaminal or foraminal and the L4 DRGs were in the foraminal region.242 The L5 DRGs were predominantly foraminal, with the possibility of being intraspinal, especially in females (not statistically significant).242,243
The lumbar DRGs are thought to play a significant role in development of low back pain and sciatica and are highly susceptible to mechanical compression.244 These pain conditions start with peripheral nerve damage, which triggers inflammatory responses within the DRG. The DRG undergoes a cascade of changes which involve activated satellite glial cells releasing proinflammatory cytokines that increase neuron excitability and recruit immune cells.245,246 DRG infiltration by macrophages, T cells and neutrophils is associated with mechanical hypersensitivity and allodynia.247,248 In addition, PNS injury induces release of neurotrophic factors that upregulate pain-related genes and DRG sensory neurons.249,250 It further alters ion channel currents leading to increased spontaneous action potential activity251 and modulates synaptic transmission leading to hyperactivity in glutamatergic transmission.252,253 These changes induce DRG neuron hyperexcitability and genetic alterations, contributing to chronic pain perception.
Lumbar DRG-S is used to treat nociceptive and neuropathic pain in the low back and lower extremities as well as pain known to be difficult to capture by traditional dorsal column SCS (eg diabetic and nondiabetic neuropathy). One of the ways it reverses neuronal hyperexcitability is through calcium-activated potassium channels in T-junctions of primary sensory neurons, which drives hyperpolarization of C-fibers membranes. By inducing action potential failure, DRG-S is postulated to amplify filtering at the T-junction and block aberrant pain signaling from reaching the central nervous system.254 In addition to modulating peripheral responses, DRG-S may have a sympatholytic effect due to the anatomic configuration of DRGs linked with the paravertebral sympathetic chain via grey (unmyelinated, efferent) and white (myelinated, afferent, C8-L2 only) communicating rami. This sympathetic-inhibiting action makes DRG-S more advantageous than neuromodulation at other neural targets, particularly in CRPS, in which an increase of alpha peripheral receptors on nociceptive fibers and endothelial dysfunction produce sympathetically mediated pain.230
DRG-S is FDA-approved for all lumbar levels. There is strong evidence for the use of lumbar DRG-S in CRPS type I and II of the lower extremity.219 The pivotal ACCURATE study was based on DRG-S predominantly at lumbar levels and demonstrated the superiority of DRG-S to thoracic SCS.231 Lumbar DRG-S may be used for select cases of diabetic or nondiabetic peripheral neuropathy. In 6 out of 12 patients with axial low back pain due to failed back surgery syndrome, DRG-S at L2 or L3 resulted in greater than 50% pain reduction at 12-months in one prospective study.255 Lumbar DRG-S has also been studied in radiculopathy, postsurgical neuropathy, and neuropathic pain of the groin/hip (L1-2), abdomen (L1), buttock (L1), pelvis (L1), knee pain (L3-4), or foot pain (L4-5).237
Thoracic Dorsal Root Ganglion Stimulation
Thoracic DRGs are mostly locate in the intervertebral foramina between the corresponding vertebrae. Little is known regarding their anatomy, positioning, and variations.256 Depending on the level, thoracic DRGs may be targeted to treat chronic refractory pain in the upper extremity (T1), trunk, or lower extremity (T11-12). Of these, DRG-S of high-thoracic spinal ganglia demands specific technical considerations. Due to anatomic distinctions, needle placement requires a more steep approach through the interlaminar space to reach the targeted neuroforamen. This approach, coupled with the presence of the spinal cord (as opposed to the cauda equina in the lumbar region) and thin CSF layer, elevates risk of spinal cord injury. Needle entered too laterally will increase the risk of ventral lead placement, leading to motor stimulation.257
The clinical significance of thoracic DRG-S often arises in postoperative or post-infectious etiologies of pain such as neuropathic pain from post-thoracotomy syndrome or post-herpetic neuralgia (PHN).233 In Post-thoracotomy syndrome, the affected level is stimulated as well as an adjacent level for support. Interestingly, DRG-S in PHN targets above and below the affected level rather than the affected level itself.258 This may be due to the observation that stimulation of the affected ganglion causes immediate increased pain, while stimulation of adjacent levels with overlapping dermatomes actually decrease pain.257 PHN is notoriously difficult to treat, and no consensus exists regarding recommendation for DRG-S as evidence is based on case studies and results are somewhat mixed.257–259
Cervical Dorsal Root Ganglion Stimulation
For certain upper extremity neuropathic or nociplastic pain, cervical DRG-S may be considered. Some possible indications may include peripheral neuropathy, brachial plexus avulsion injuries, compression neuropathies, mononeuropathy (eg median, ulnar, radial) and CRPS type I and II of the upper extremities.260 Compared to SCS, DRG-S may offer more precise targeting in the treatment of hand and upper extremity pain through more specific dermatomal coverage with potentially less variability in intensity.
In CRPS of the upper limbs, the heightened pain signals in the upper extremities travel from the peripheral receptive field to the DRG. This surge in pain afferents drive changes in the expression and function of sodium channels. Additionally, there is an increased production of neuroinflammatory substances that modify membrane excitability. This alteration results in the generation of ectopic and spontaneous action potentials across all DRG neurons connected to the peripheral nerves of the brachial plexus (C5-T1), a phenomenon known as convergence.230
C8 and T1 DRG-S appears to an optimal approach for addressing CRPS affecting the hand and wrist, based on several factors: First, pain signals from the hand and wrist travel through peripheral nerves to the brachial plexus (C5-T1), thereby crosstalk phenomenon is more likely between DRGs of the same brachial plexus rather than with those at lower levels. Additionally, the C8-T1 level, which includes the stellate ganglion, possesses a higher concentration of sympathetic fibers compared to levels above, making it more conducive to a sympatholytic effect than a superior level.261,262
It is important to note that the location of the cervical DRG, in relation to the pedicle, particularly varies between levels. The cervical DRG are found foraminal in close proximity to its respective nerve root. The distance between the thecal sac and the nerve root increases when descending from the upper to lower cervical nerve roots.263 Thus, the location of the DRG tends to be more lateral than the level above. Also, due to the high mobility of the cervical spine, cervical DRG-S carries increased risk of lead migration.257
Evidence on cervical DRG-S is sparse and based on case reports or series. Currently, there are no FDA-approved cervical levels for DRG-S. In Europe, DRG-S received CE mark since 2011 and has no anatomical limitations.219 Considering recent recommendations emphasizing best practices for cervical neurostimulation, it is advised to limit DRG-S to the lower cervical spine due to increased risk of vascular and neurologic injuries.264 Upper cervical levels are generally more difficult to safely target due to the smaller diameter of the central canal.263 Moreover, the vertebral artery bisects the transverse process canal (coursing ventral to the DRG) above C7 level.265 The ligamentum flavum may also be absent above C6.266,267 NACC recommends a conservative approach, advocating for cervical epidural entry for lead placement at C6 or lower.264 Further research is needed to assess the efficacy of cervical DRG-S in treating upper extremity pain.
Frequency of DRG Stimulation
The DRG is a coalition of cell bodies belonging to primary sensory neurons. The axons of these sensory neurons encompass a diversity of fibers with varying sizes and excitability, including Aβ, Aδ, and C fibers.220 A key mechanism of pain relief from DRG-S involves the preferential activation of Aβ, Aδ, and C fibers at low frequencies. By producing action potentials at extremely low frequencies, DRG-S effectively blocks pain stimuli through the activation of opioid receptors without involving the GABA system.268 Pain alleviation appears to be a result of multiple mechanisms, encompassing various factors such as the activation of inhibitory interneurons in the dorsal horn, as proposed by the gate-control theory.269
Frequency is defined by the number of pulses that are applied in a given time duration. Traditional (tonic) SCS leads are placed in the dorsal epidural space and employs continuous stimulation within the frequency range of 40–100 hz. The orthodromic stimulation at the dorsal columns generate paresthesias in dermatomes corresponding to the patient’s pain location. This stimulation involves pulse widths on the order of hundreds of microseconds. In contrast, DRG-S is commonly programmed at frequency range of 10–50 hz, with pulse widths between 30 to 1000 microseconds and an amplitude around 1 mA.234 The ACCURATE study utilized a setting of frequency at or above 16 hz and average pulse width of 260 microseconds.
Recent work on very low frequency (<5 hz) DRG-S have theorized low-threshold mechanoreceptor (LTMR) mediated inhibition as another primary mechanism of DRG-S.270 The DRG houses not only nociceptive fibers but also non-nociceptive LTMR fibers that carry light touch and mechanosensation afferents.271 LTMRs fire frequencies of 0.5–5 Hz272 and are modulated by endorphins273 At very low frequencies, DRG-S is thought to selectively stimulate LTMR fibers to modulate and transmit pain.268 In a prospective study of 20 patients, DRG-S frequency was tapered to 4 hz without loss of pain relief at 17 weeks.274
Waveforms and Amplitude
Neuromodulation devices mostly utilize square waves that are delivered at a constant frequency and fixed pulse width. These waves are administered either continuously without interruption, or in grouped intervals known as “bursts.” During programming sessions, amplitudes are typically adjusted based on patient feedback or previous anecdotal data indicating which amplitude level has been helpful.275
The amplitude refers to the current when in constant current mode, and voltage when in constant voltage mode. SCS induces paresthesias when applied with sufficient amplitude. In high-frequency SCS (commercially known as HF10), which tends to be paresthesia-free, delivers an amplitude of 1–5 mA, which is below the paresthesia threshold unlike in tonic SCS. Similarly, DRG-S is typically programmed at amplitude around 1mA. The notably lower amplitude of DRG-S compared to tonic SCS appears to be related to the proximal location of the electrode to the site of action and the relative lack of CSF.234
The diverse waveforms available in SCS has not yet been applied in DRG-S, which is a relatively new technology. The different SCS waveforms operates through a variety of mechanisms, implicating the engagement of the wide dynamic neurons in the dorsal spinal cord, activation of Aβ fibers, supraspinal effects, and both antegrade and retrograde influences. Overall, it is observed that different waveforms exhibit distinct behaviors. An exact understanding of these processes, while considering the fiber threshold hypothesis, remains unclear.275 Currently, only tonic DRG-S is used clinically. However, a preclinical study using rats demonstrated that burst stimulation of L5 DRG was equal in efficacy to conventional DRG-S in a model of painful diabetic peripheral neuropathy.276 As our understanding of the pathophysiology of pain and mechanisms of DRG-S improves, tailoring waveforms to specific pathways can optimize treatment strategies.
Conclusions
Chronic pain presents several major clinical, social and economic setbacks and has therefore been the focus of ongoing global research. A large number of studies has been undertaken in the understanding of the pain pathways and the pathophysiology of chronic pain. Such pathways are complex processes with multiple interdependent structures controlled by multiple modulatory systems consisting of neuronal, inflammatory and endocrinological components. These processes are further complicated by the fact that chronic pain is a highly heterogeneous disorder and most systemic treatments are associated with significant side effects. The aim of chronic pain management should therefore focus on prevention, as the consequent sensitization of pain pathways results in a positive feedback loop and multiple pathological changes at peripheral, spinal and supra-spinal levels. Treatment goals should target precise portions of the pain pathways by way of disease modifying therapies to eradicate any contributing peripheral and central sensitizations.
While the genetics of pain have been a hot topic in recent years, what has also emerged has shown that there are several distinct neural circuits associated with pain that can potentially be disrupted and thus utilized. Neuromodulation techniques leverage these existing neural circuits to create therapies to intervene upon the pathways which carry pain signals. As new therapies evolve, there will be a major need to tailor therapy utilization, dosing and targeting to the neural response created by the delivered electrical current. These therapies continue to offer hope to both the patients suffering from these conditions as well as tools for physicians who face the constant challenge of improving patient suffering, function and quality of life.
Disclosure
Dr Alaa Abd-Elsayed is a consultant for Medtronic and Curonix. Dr Mansoor M Aman is a consultant for Abbott and Medtronic. Dr Mark Malinowski reports personal fees from Abbott, Biotronik NEURO, SI Bone, Inc, Medtronic, and Nalu Medical. Dr Usman Latif reports grants and/or personal fees from Nalu Medical, SPR, Abbott, Nevro, Vertos, Spinal Simplicity, Omnia Medical, Brixton Biosciences, InFormed Consent, and Mainstay Medical, outside the submitted work. Dr David Dickerson reports grants and/or personal fees from Abbott, SPR, Biotronik, Vertos, and Vertex, outside the submitted work. Dr Timothy Lubenow reports grants and/or personal fees from Abbott, Boston Scientific, Pen Tec, PainTeq, Biotronik. Dr Michael Farrell II reports personal fees from Abbott, outside the submitted work. Dr Dawood Sayed reports personal fees and options from Painteq; personal fees from Saluda and Abbott; options from Mainstay and Surgentec, outside the submitted work. Dr Timothy Deer reports personal fees for consultancy and research from Abbott and Saluda, during the conduct of the study; personal fees for consultancy or research from Vertos, SpineThera, Mainstay, Cornerloc, Boston Scientific, PainTeq, Spinal Simplicity, SPR Therapeutics, Biotronik, Aurora, and Nervonic, outside the submitted work. In addition, Dr Timothy Deer has a patent pending to Abbott. The authors report no other conflicts of interest in this work.
References
- 1.Wallig MA, Bolon B, Haschek WM, Rousseaux CG. Fundamentals of Toxicologic Pathology. Academic press; 2017. [Google Scholar]
- 2.Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018;19(8):2164. doi: 10.3390/ijms19082164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterweis M, Kleinman A, Mechanic D. Pain and disability: clinical, behavioral, and public policy perspectives. 1987. [PubMed]
- 4.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019 [DOI] [PubMed] [Google Scholar]
- 5.Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Primary Care. 2012;39(3):561–571. doi: 10.1016/j.pop.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gildenberg PL. History of Electrical Neuromodulation for Chronic Pain. Malden, USA: Blackwell Publishing Inc; 2006:S7–S13. [Google Scholar]
- 7.Melzack R, Wall PD. Pain mechanisms: a new theory: a gate control system modulates sensory input from the skin before it evokes pain perception and response. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- 8.North RB, Lempka SF, Guan Y, et al. Glossary of neurostimulation terminology: a collaborative neuromodulation foundation, institute of neuromodulation, and international neuromodulation society project. Neuromodulation. 2022;25(7):1050–1058. doi: 10.1016/j.neurom.2021.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Cruccu G, Aziz T, Garcia‐Larrea L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14(9):952–970. doi: 10.1111/j.1468-1331.2007.01916.x [DOI] [PubMed] [Google Scholar]
- 10.Kumar K, Rizvi S. Historical and present state of neuromodulation in chronic pain. Curr Pain Headache Rep. 2014;18:1–7. doi: 10.1007/s11916-013-0387-y [DOI] [PubMed] [Google Scholar]
- 11.Denison T, Morrell MJ. Neuromodulation in 2035: the neurology future forecasting series. Neurology. 2022;98(2):65–72. doi: 10.1212/WNL.0000000000013061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provenzano DA, Heller JA, Hanes MC. Current perspectives on neurostimulation for the management of chronic low back pain: a narrative review. J Pain Res. 2021;14:463–479. doi: 10.2147/JPR.S249580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karri J, Orhurhu V, Wahezi S, Tang T, Deer T, Abd-Elsayed A. Comparison of spinal cord stimulation waveforms for treating chronic low back pain: systematic review and meta-analysis. Pain Physician. 2020;23(5):451. [PubMed] [Google Scholar]
- 14.Purves D, Augustine G, Fitzpatrick D, et al. Neuroscience 2nd edition. In: Types of Eye Movements and Their Functions. Sunderland (MA): Sinauer Associates; 2001. [Google Scholar]
- 15.Grider MH, Jessu R, Kabir R. Physiology, action potential. 2019. [PubMed]
- 16.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760–3772. doi: 10.1172/JCI42843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vance CG, Dailey DL, Chimenti RL, Van Gorp BJ, Crofford LJ, Sluka KA. Using TENS for pain control: update on the state of the evidence. Medicina. 2022;58(10):1332. doi: 10.3390/medicina58101332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minett MS, Falk S, Santana-Varela S, et al. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 2014;6(2):301–312. doi: 10.1016/j.celrep.2013.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra S, Tadi P. Neuroanatomy, nucleus gracilis. 2019. [PubMed]
- 20.Hodge CJ, Apkarian A. The spinothalamic tract. Crit Rev Neurobiol. 1990;5(4):363–397. [PubMed] [Google Scholar]
- 21.Willis W, Westlund K. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14(1):2. doi: 10.1097/00004691-199701000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan W, Sullivan SJ, Sdrulla AD. Dorsal column and root stimulation at Aβ-fiber intensity activate superficial dorsal horn glutamatergic and GABAergic populations. Molecular Pain. 2022;18:17448069221079559. doi: 10.1177/17448069221079559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams FG, Mullet MA, Beitz AJ. Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res. 1995;690(2):207–216. doi: 10.1016/0006-8993(95)00554-4 [DOI] [PubMed] [Google Scholar]
- 24.Bagley EE, Ingram SL. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology. 2020;173:108131. doi: 10.1016/j.neuropharm.2020.108131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlereth T. Guideline “diagnosis and non interventional therapy of neuropathic pain” of the German Society of Neurology (deutsche Gesellschaft für Neurologie). Neurol Res Pract. 2020;2(1):1–19. doi: 10.1186/s42466-020-00063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Zou W, Lee K, Wang E, Zhu X, Guo Q. Different symptoms of neuropathic pain can be induced by different degrees of compressive force on the C7 dorsal root of rats. Spine J. 2012;12(12):1154–1160. doi: 10.1016/j.spinee.2012.10.036 [DOI] [PubMed] [Google Scholar]
- 27.Doshi TL, Dworkin RH, Polomano RC, et al. AAAPT diagnostic criteria for acute neuropathic pain. Pain Med. 2021;22(3):616–636. doi: 10.1093/pm/pnaa407 [DOI] [PubMed] [Google Scholar]
- 28.Rowbotham MC. Tricyclic antidepressants and opioids—better together? Pain. 2015;156(8):1373–1374. doi: 10.1097/j.pain.0000000000000189 [DOI] [PubMed] [Google Scholar]
- 29.Fava M, Mulroy R, Alpert J, Nierenberg AA, Rosenbaum JF. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760–1762. doi: 10.1176/ajp.154.12.1760 [DOI] [PubMed] [Google Scholar]
- 30.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. doi: 10.1016/S1474-4422(14)70102-4 [DOI] [PubMed] [Google Scholar]
- 31.Turan A, Karamanlıoğlu B, Memiş D, et al. Analgesic effects of gabapentin after spinal surgery. J Am Soc Anesthesiologists. 2004;100(4):935–938. [DOI] [PubMed] [Google Scholar]
- 32.Dworkin RH, O’Connor AB, Kent J, et al. Interventional management of neuropathic pain: neuPSIG recommendations. Pain®. 2013;154(11):2249–2261. doi: 10.1016/j.pain.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinu P, Morsy MA, Nair AB, et al. Novel therapies for the treatment of neuropathic pain: potential and pitfalls. J Clin Med. 2022;11(11):3002. doi: 10.3390/jcm11113002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prescott SA, Ratté S. Somatosensation and pain. In: Conn’s Translational Neuroscience. Elsevier; 2017:517–539. [Google Scholar]
- 35.Tominaga M. Thermal sensation (cold and heat) through thermosensitive TRP channel activation. 2008.
- 36.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89(2):707–758. doi: 10.1152/physrev.00025.2008 [DOI] [PubMed] [Google Scholar]
- 37.Mylius V, Lloret SP, Cury RG, et al. The Parkinson disease pain classification system: results from an international mechanism-based classification approach. Pain. 2021;162(4):1201. doi: 10.1097/j.pain.0000000000002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- 39.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perl ER, Kumazawa T, Lynn B, Kenins P. Sensitization of high threshold receptors with unmyelinated (C) afferent fibers. Prog Brain Res. 1976;43:263–277. doi: 10.1016/S0079-6123(08)64359-9 [DOI] [PubMed] [Google Scholar]
- 41.Wei S, Tao Z, Xue Y, Cao D. Peripheral Sensitization. In: Peripheral Nerve Disorders and Treatment. IntechOpen; 2019. [Google Scholar]
- 42.Vardeh D, Naranjo JF. Peripheral and central sensitization. In: Yong R, Nguyen M, Nelson E, Urman R, editors. Pain Medicine. Springer; 2017:15–17. [Google Scholar]
- 43.Bhave G, Gereau RW IV. Posttranslational mechanisms of peripheral sensitization. J Neurobiol. 2004;61(1):88–106. doi: 10.1002/neu.20083 [DOI] [PubMed] [Google Scholar]
- 44.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4 [DOI] [PubMed] [Google Scholar]
- 45.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 46.Dai Y. TRPs and pain. Semin Immunopathol. 2016;38(3):277–291. doi: 10.1007/s00281-015-0526-0 [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Gu L, Ruan Y, et al. Pirt together with TRPV1 is involved in the regulation of neuropathic pain. Neural Plast. 2018;2018:4861491. doi: 10.1155/2018/4861491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okun A, DeFelice M, Eyde N, et al. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warwick CA, Shutov LP, Shepherd AJ, Mohapatra DP, Usachev YM. Mechanisms underlying mechanical sensitization induced by complement C5a: the roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain. 2019;160(3):702–711. doi: 10.1097/j.pain.0000000000001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz B, Zaguri R, Edvardson S, et al. Nociception and pain in humans lacking a functional TRPV1 channel. J Clin Invest. 2023;133(3). doi: 10.1172/JCI153558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toledo-Aral JJ, Moss BL, He ZJ, et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci U S A. 1997;94(4):1527–1532. doi: 10.1073/pnas.94.4.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black JA, Frezel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain. 2012;8:82. doi: 10.1186/1744-8069-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang W, Berta T, Kim YH, Lee S, Lee SY, Ji RR. Expression and role of voltage-gated sodium channels in human dorsal root ganglion neurons with special focus on Nav1.7, species differences, and regulation by paclitaxel. Neurosci Bull. 2018;34(1):4–12. doi: 10.1007/s12264-017-0132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann N, King T, Murphy R. Review of primary and secondary erythromelalgia. Clin Exp Dermatol. 2019;44(5):477–482. doi: 10.1111/ced.13891 [DOI] [PubMed] [Google Scholar]
- 55.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 56.Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94(1):81–140. doi: 10.1152/physrev.00023.2013 [DOI] [PubMed] [Google Scholar]
- 57.Kim C, Jun K, Lee T, et al. Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol Cell Neurosci. 2001;18(2):235–245. doi: 10.1006/mcne.2001.1013 [DOI] [PubMed] [Google Scholar]
- 58.Saegusa H, Kurihara T, Zong S, et al. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20(10):2349–2356. doi: 10.1093/emboj/20.10.2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanegas H, Schaible H. Effects of antagonists to high-threshold calcium channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain. 2000;85(1–2):9–18. doi: 10.1016/s0304-3959(99)00241-9 [DOI] [PubMed] [Google Scholar]
- 60.Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol. 2011;163(3):484–495. doi: 10.1111/j.1476-5381.2011.01256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joksimovic SL, Joksimovic SM, Tesic V, et al. Selective inhibition of Ca(V)3.2 channels reverses hyperexcitability of peripheral nociceptors and alleviates postsurgical pain. Sci Signal. 2018;11(545). doi: 10.1126/scisignal.aao4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shao PP, Ye F, Chakravarty PK, et al. Aminopiperidine sulfonamide Cav2.2 channel inhibitors for the treatment of chronic pain. J Med Chem. 2012;55(22):9847–9855. doi: 10.1021/jm301056k [DOI] [PubMed] [Google Scholar]
- 63.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LaMotte RH, Thalhammer JG, Torebjork HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2(6):765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38(4):397–421. doi: 10.1016/0301-0082(92)90027-c [DOI] [PubMed] [Google Scholar]
- 66.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765 [DOI] [PubMed] [Google Scholar]
- 67.Nijs J, George SZ, Clauw DJ, et al. Central sensitization in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3:e383–92. doi: 10.1016/S2665-9913(21)00032-1 [DOI] [PubMed] [Google Scholar]
- 68.Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum. 2014;44(1):68–75. doi: 10.1016/j.semarthrit.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 69.Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain. 2013;17(3):299–312. doi: 10.1002/j.1532-2149.2012.00193.x [DOI] [PubMed] [Google Scholar]
- 70.Thompson SW, Woolf CJ, Sivilotti LG. Small-caliber afferent inputs produce a heterosynaptic facilitation of the synaptic responses evoked by primary afferent A-fibers in the neonatal rat spinal cord in vitro. J Neurophysiol. 1993;69(6):2116–2128. doi: 10.1152/jn.1993.69.6.2116 [DOI] [PubMed] [Google Scholar]
- 71.Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep. 2016;20(4):25. doi: 10.1007/s11916-016-0556-x [DOI] [PubMed] [Google Scholar]
- 72.Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422(3):464–487. doi: [DOI] [PubMed] [Google Scholar]
- 73.Antal M, Fukazawa Y, Eordogh M, et al. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci. 2008;28(39):9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. Pain. 1995;61(3):383–390. doi: 10.1016/0304-3959(94)00195-K [DOI] [PubMed] [Google Scholar]
- 75.Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44(3):293–299. doi: 10.1016/0304-3959(91)90100-C [DOI] [PubMed] [Google Scholar]
- 76.Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z. Spinal substance P and N-methyl-D-aspartate receptors are coactivated in the induction of central sensitization of the nociceptive flexor reflex. Neuroscience. 1992;51(3):641–648. doi: 10.1016/0306-4522(92)90303-j [DOI] [PubMed] [Google Scholar]
- 77.Gyorfi M, Rupp A, Abd-Elsayed A. Fibromyalgia Pathophysiology. Biomedicines. 2022;10(12):3070. doi: 10.3390/biomedicines10123070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8(2):143–151. doi: 10.1097/SPC.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarzi-Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16(11):645–660. doi: 10.1038/s41584-020-00506-w [DOI] [PubMed] [Google Scholar]
- 80.Jensen KB, Loitoile R, Kosek E, et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsuda M, Huh Y, Ji R-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131–139. doi: 10.1007/s00540-018-2579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vasic V, Schmidt MH. Resilience and vulnerability to pain and inflammation in the hippocampus. Int J Mol Sci. 2017;18(4):739. doi: 10.3390/ijms18040739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15(1):43–53. doi: 10.1038/nrn3617 [DOI] [PubMed] [Google Scholar]
- 84.De Ridder D, Adhia D, Vanneste S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev. 2021;130:125–146. [DOI] [PubMed] [Google Scholar]
- 85.Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131(9):2387–2400. doi: 10.1093/brain/awn169 [DOI] [PubMed] [Google Scholar]
- 86.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581. doi: 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- 87.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. doi: 10.1152/physrev.00031.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benzon H, Raja SN, Fishman SE, Liu SS, Cohen SP. Essentials of Pain Medicine E-Book. Elsevier Health Sciences; 2011. [Google Scholar]
- 89.Seybold VS. The role of peptides in central sensitization. Sens Nerves. 2009:451–491. [DOI] [PubMed] [Google Scholar]
- 90.Russell FA, King R, Smillie S-J, Kodji X, Brain S. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi: 10.1152/physrev.00034.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaplan AP, Joseph K, Silverberg M. Pathways for bradykinin formation and inflammatory disease. J Allergy Clin Immunol. 2002;109(2):195–209. doi: 10.1067/mai.2002.121316 [DOI] [PubMed] [Google Scholar]
- 92.Pethő G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699–1775. doi: 10.1152/physrev.00048.2010 [DOI] [PubMed] [Google Scholar]
- 93.Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Excitation and sensitization of nociceptors by bradykinin: what do we know? Exp Brain Res. 2009;196:53–65. doi: 10.1007/s00221-009-1814-5 [DOI] [PubMed] [Google Scholar]
- 94.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275(18):4448–4455. doi: 10.1111/j.1742-4658.2008.06580.x [DOI] [PubMed] [Google Scholar]
- 95.Cortes-Burgos LA, Zweifel BS, Settle SL, et al. CJ-13610, an orally active inhibitor of 5-lipoxygenase is efficacious in preclinical models of pain. Eur J Pharmacol. 2009;617(1–3):59–67. doi: 10.1016/j.ejphar.2009.06.058 [DOI] [PubMed] [Google Scholar]
- 96.Skaper SD. Nerve growth factor: a neurokine orchestrating neuroimmune-endocrine functions. Mol Neurobiol. 2001;24:183–199. doi: 10.1385/MN:24:1-3:183 [DOI] [PubMed] [Google Scholar]
- 97.Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338:1–18. doi: 10.1016/j.neuroscience.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 98.Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21(17):6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dussor G. ASICs as therapeutic targets for migraine. Neuropharmacology. 2015;94:64–71. doi: 10.1016/j.neuropharm.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sluka KA, Gregory NS. The dichotomized role for acid sensing ion channels in musculoskeletal pain and inflammation. Neuropharmacology. 2015;94:58–63. doi: 10.1016/j.neuropharm.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 2011;130(3):283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun J, Chen S-R, Pan H-L. μ-Opioid receptors in primary sensory neurons are involved in supraspinal opioid analgesia. Brain Res. 2020;1729:146623. doi: 10.1016/j.brainres.2019.146623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Desroches J, Charron S, Bouchard J-F, Beaulieu P. Endocannabinoids decrease neuropathic pain-related behavior in mice through the activation of one or both peripheral CB1 and CB2 receptors. Neuropharmacology. 2014;77:441–452. doi: 10.1016/j.neuropharm.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 104.Afridi B, Khan H, Akkol EK, Aschner M. Pain perception and management: where do we stand? Curr Mol Pharmacol. 2021;14(5):678. doi: 10.2174/1874467213666200611142438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pace MC, Passavanti MB, De Nardis L, et al. Nociceptor plasticity: a closer look. J Cell Physiol. 2018;233(4):2824–2838. doi: 10.1002/jcp.25993 [DOI] [PubMed] [Google Scholar]
- 107.Baronio M, Sadia H, Paolacci S, et al. Molecular aspects of regional pain syndrome. Pain Res Manag. 2020;2020:1–10. doi: 10.1155/2020/7697214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee GI, Neumeister MW. Pain: pathways and physiology. Clin Plast Surg. 2020;47(2):173–180. doi: 10.1016/j.cps.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 109.Schaible H-G. Peripheral and central mechanisms of pain generation. Analgesia. 2007:3–28. [DOI] [PubMed] [Google Scholar]
- 110.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Świeboda P, Filip R, Prystupa A, Drozd M. Assessment of pain: types, mechanism and treatment. Pain. 2013;2(7):2–7. [PubMed] [Google Scholar]
- 112.Boddeke EW. Involvement of chemokines in pain. Eur J Pharmacol. 2001;429(1–3):115–119. doi: 10.1016/S0014-2999(01)01311-5 [DOI] [PubMed] [Google Scholar]
- 113.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. [DOI] [PubMed] [Google Scholar]
- 114.Okuse K. Pain signalling pathways: from cytokines to ion channels. Int J Biochem Cell Biol. 2007;39(3):490–496. doi: 10.1016/j.biocel.2006.11.016 [DOI] [PubMed] [Google Scholar]
- 115.Messlinger K, Handwerker H. Physiology of pain. Der Schmerz. 2015;29:522–530. [DOI] [PubMed] [Google Scholar]
- 116.Sneddon LU. Comparative physiology of nociception and pain. Physiology. 2017. [DOI] [PubMed]
- 117.Ringkamp M, Dougherty PM, Raja SN. Anatomy and physiology of the pain signaling process. In: Essentials of Pain Medicine. Elsevier; 2018:3–10.e1. [Google Scholar]
- 118.Treede R-D, Jensen T, Campbell J, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 119.Mercer Lindsay N, Chen C, Gilam G, Mackey S, Scherrer G. Brain circuits for pain and its treatment. Sci Transl Med. 2021;13(619):eabj7360. doi: 10.1126/scitranslmed.abj7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Renthal W, Tochitsky I, Yang L, et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron. 2020;108(1):128–144.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dafny N. Pain tracts and sources. Electronic Textbook for the Neurosciences Neuroscience Online. 2020.
- 122.Al-Chalabi M, Reddy V, Gupta S. Neuroanatomy, spinothalamic tract. 2018. [PubMed]
- 123.Murphy Z. Ascending tracts/spinothalamic tract. Ninja Nerd. Available from: https://ninjanerd.org. Accessed November 7, 2024. [Google Scholar]
- 124.Encyclopedia. WH. Paleospinothalamic Tract.
- 125.Dubuc B. The brain from top to bottom. 2002.
- 126.Bonica JJ. A necessidade de uma taxonomia. Braz J Anesthesiol. 2020;30(5):349–351. Brazilian Portugese. [Google Scholar]
- 127.Burgess PR, Perl E. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marshall TM, Herman DS, Largent-Milnes TM, et al. Activation of descending pain-facilitatory pathways from the rostral ventromedial medulla by cholecystokinin elicits release of prostaglandin-E2 in the spinal cord. Pain. 2012;153(1):86–94. doi: 10.1016/j.pain.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem Pharmacol. 2015;97(4):590–600. doi: 10.1016/j.bcp.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khodorova A, Montmayeur J-P, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10(1):4–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vincler M, McIntosh JM. Targeting the α9α10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin Ther Targets. 2007;11(7):891–897. doi: 10.1517/14728222.11.7.891 [DOI] [PubMed] [Google Scholar]
- 132.Zhang W, Gardell S, Zhang D, et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132(3):778–787. doi: 10.1093/brain/awn330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nicoletti M, Neri G, Maccauro G, et al. Impact and neuropeptide substance Pan inflammatory compound on arachidonic acid compound generation. Inter J Immuno Pharmacol. 2012;25(4):849–857. doi: 10.1177/039463201202500403 [DOI] [PubMed] [Google Scholar]
- 134.Elsenbruch S, Icenhour A, Enck P. Visceral pain–a biopsychological perspective. e-Neuroforum. 2017;23(3):105–110. doi: 10.1515/nf-2017-A029 [DOI] [Google Scholar]
- 135.Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bourne S, Machado AG, Nagel SJ. Basic anatomy and physiology of pain pathways. Neurosurg Clinics. 2014;25(4):629–638. doi: 10.1016/j.nec.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 137.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088 [DOI] [PubMed] [Google Scholar]
- 138.Reddi D, Curran N, Stephens R. An introduction to pain pathways and mechanisms. Br J Hosp Med. 2013;74(Sup12):C188–C191. doi: 10.12968/hmed.2013.74.Sup12.C188 [DOI] [PubMed] [Google Scholar]
- 139.Steeds CE. The anatomy and physiology of pain. Surgery. 2009;27(12):507–511. [Google Scholar]
- 140.Laight D. Overview of peripheral nervous system pharmacology. Nurse Prescribing. 2013;11(9):448–454. doi: 10.12968/npre.2013.11.9.448 [DOI] [Google Scholar]
- 141.Raja SN, Dougherty PM. Anatomy and physiology of somatosensory and pain processing. In: Essentials of Pain Medicine. Elsevier; 2004:1–6. [Google Scholar]
- 142.Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based approach to pain diagnosis. J Pain. 2016;17(9):T50–T69. doi: 10.1016/j.jpain.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fenton BW, Shih E, Zolton J. The neurobiology of pain perception in normal and persistent pain. Pain Manag. 2015;5(4):297–317. doi: 10.2217/pmt.15.27 [DOI] [PubMed] [Google Scholar]
- 144.Xiang Y, Wang Y, Gao S, Zhang X, Cui R. Neural mechanisms with respect to different paradigms and relevant regulatory factors in empathy for pain. Front Neurosci. 2018;12:507. doi: 10.3389/fnins.2018.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Talbot K, Madden V, Jones S, Moseley G. The sensory and affective components of pain: are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br J Anaesth. 2019;123(2):e263–e272. doi: 10.1016/j.bja.2019.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Singh A, Patel D, Li A, et al. Mapping cortical integration of sensory and affective pain pathways. Curr Biol. 2020;30(9):1703–1715.e5. doi: 10.1016/j.cub.2020.02.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang S, Chang MC. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci. 2019;20(13):3130. doi: 10.3390/ijms20133130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Therapy. 2007;9(4):1–7. doi: 10.1186/ar2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Greening J, Lynn B, Leary R. Sensory and autonomic function in the hands of patients with non-specific arm pain (NSAP) and asymptomatic office workers. Pain. 2003;104(1–2):275–281. doi: 10.1016/S0304-3959(03)00010-1 [DOI] [PubMed] [Google Scholar]
- 150.Gockel M, Lindholm H, Niemistö L, Hurri H. Perceived disability but not pain is connected with autonomic nervous function among patients with chronic low back pain. J Rehabil Med. 2008;40(5):355–358. doi: 10.2340/16501977-0172 [DOI] [PubMed] [Google Scholar]
- 151.Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromolecular Med. 2008;10:141–147. doi: 10.1007/s12017-007-8018-6 [DOI] [PubMed] [Google Scholar]
- 152.Queme LF, Ross JL, Jankowski MP. Peripheral mechanisms of ischemic myalgia. Front Cell Neurosci. 2017;11:419. doi: 10.3389/fncel.2017.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mansour A, Farmer M, Baliki M, Apkarian AV. Chronic pain: the role of learning and brain plasticity. Restorat Neurol Neurosci. 2014;32(1):129–139. doi: 10.3233/RNN-139003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ong Sio LC, Hom B, Garg S, Abd-Elsayed A. Mechanism of action of peripheral nerve stimulation for chronic pain: a narrative review. Int J Mol Sci. 2023;24(5):4540. doi: 10.3390/ijms24054540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ottestad E, Orlovich DS. History of peripheral nerve stimulation—update for the 21st century. Pain Med. 2020;21(Supplement_1):S3–S5. doi: 10.1093/pm/pnaa165 [DOI] [PubMed] [Google Scholar]
- 156.Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2(3):217–221. doi: 10.1046/j.1525-1403.1999.00217.x [DOI] [PubMed] [Google Scholar]
- 157.Milby AH, Halpern CH, Baltuch GH. Vagus nerve stimulation for epilepsy and depression. Neurotherapeutics. 2008;5:75–85. doi: 10.1016/j.nurt.2007.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Z-H, Liu Z-H. Treatment for overactive bladder: a meta-analysis of tibial versus parasacral neuromodulation. Medicine. 2022;101(41). doi: 10.1097/MD.0000000000031165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Strand N, D’Souza RS, Hagedorn JM, et al. Evidence-based clinical guidelines from the American Society of Pain and Neuroscience for the use of implantable peripheral nerve stimulation in the treatment of chronic pain. J Pain Res. 2022;15:2483–2504. doi: 10.2147/JPR.S362204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jeong Y, Baik EJ, Nam TS, Paik KS. Effects of iontophoretically applied naloxone, picrotoxin and strychnine on dorsal horn neuron activities treated with high frequency conditioning stimulation in cats. Yonsei Med J. 1995;36(4):336–347. doi: 10.3349/ymj.1995.36.4.336 [DOI] [PubMed] [Google Scholar]
- 161.Torebjörk HE, Hallin RG. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg. 1974;37(6):653. doi: 10.1136/jnnp.37.6.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Deer TR, Eldabe S, Falowski SM, et al. Peripherally induced reconditioning of the central nervous system: a proposed mechanistic theory for sustained relief of chronic pain with percutaneous peripheral nerve stimulation. J Pain Res. 2021;14:721–736. doi: 10.2147/JPR.S297091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Papuć E, Rejdak K. The role of neurostimulation in the treatment of neuropathic pain. Ann Agric Environ Med. 2013;1:14–17. [PubMed] [Google Scholar]
- 164.Lin T, Gargya A, Singh H, Sivanesan E, Gulati A. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med. 2020;21(Supplement_1):S6–S12. doi: 10.1093/pm/pnaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liao C-F, Hsu S-T, Chen C-C, et al. Effects of electrical stimulation on peripheral nerve regeneration in a silicone rubber conduit in taxol-treated rats. Materials. 2020;13(5):1063. doi: 10.3390/ma13051063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ahmed S, Plazier M, Ost J, et al. The effect of occipital nerve field stimulation on the descending pain pathway in patients with fibromyalgia: a water PET and EEG imaging study. BMC Neurol. 2018;18(1):1–10. doi: 10.1186/s12883-018-1190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bandeira JS, Antunes LDC, Soldatelli MD, Sato JR, Fregni F, Caumo W. Functional spectroscopy mapping of pain processing cortical areas during non-painful peripheral electrical stimulation of the accessory spinal nerve. Front Human Neurosci. 2019;13:200. doi: 10.3389/fnhum.2019.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meyer-Frießem CH, Wiegand T, Eitner L, et al. Effects of spinal cord and peripheral nerve stimulation reflected in sensory profiles and endogenous pain modulation. Clin J Pain. 2019;35(2):111–120. doi: 10.1097/AJP.0000000000000661 [DOI] [PubMed] [Google Scholar]
- 169.García-Magro N, Negredo P, Martin YB, Á N, Avendaño C. Modulation of mechanosensory vibrissal responses in the trigeminocervical complex by stimulation of the greater occipital nerve in a rat model of trigeminal neuropathic pain. J Headache Pain. 2020;21:1–17. doi: 10.1186/s10194-020-01161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Men D-S, Matsui Y. Peripheral nerve stimulation increases serotonin and dopamine metabolites in rat spinal cord. Brain Res Bull. 1994;33(6):625–632. doi: 10.1016/0361-9230(94)90225-9 [DOI] [PubMed] [Google Scholar]
- 171.Schaible HG, Hope P, Lang C, Duggan A. Calcitonin gene‐related peptide causes intraspinal spreading of substance P released by peripheral stimulation. Eur J Neurosci. 1992;4(8):750–757. doi: 10.1111/j.1460-9568.1992.tb00184.x [DOI] [PubMed] [Google Scholar]
- 172.Sirls LT, Schonhoff A, Waldvogel A, Peters KM. Development of an implant technique and early experience using a novel implantable pulse generator with a quadripolar electrode array at the tibial nerve for refractory overactive bladder. Neurourol Urodynamics. 2023;42(2):427–435. doi: 10.1002/nau.25117 [DOI] [PubMed] [Google Scholar]
- 173.Yecies T, Li S, Zhang Y, et al. Spinal interneuronal mechanisms underlying pudendal and tibial neuromodulation of bladder function in cats. Exp Neurol. 2018;308:100–110. doi: 10.1016/j.expneurol.2018.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63(1):82–104. doi: 10.1152/jn.1990.63.1.82 [DOI] [PubMed] [Google Scholar]
- 175.Yang F, Carteret A, Wacnik P, et al. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–480. doi: 10.1016/j.neuroscience.2011.09.049 [DOI] [PubMed] [Google Scholar]
- 176.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149 [DOI] [PubMed] [Google Scholar]
- 177.Deer TR, Esposito MF, McRoberts WP, et al. A systematic literature review of peripheral nerve stimulation therapies for the treatment of pain. Pain Med. 2020;21(8):1590–1603. doi: 10.1093/pm/pnaa030 [DOI] [PubMed] [Google Scholar]
- 178.Gilligan C, Volschenk W, Russo M, et al. Three-year durability of restorative neurostimulation effectiveness in patients with chronic low back pain and multifidus muscle dysfunction. Neuromodulation. 2023;26(1):98–108. doi: 10.1016/j.neurom.2022.08.457 [DOI] [PubMed] [Google Scholar]
- 179.Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2020;45(1):44–51. doi: 10.1136/rapm-2019-100937 [DOI] [PubMed] [Google Scholar]
- 180.Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared to usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(1):17. doi: 10.1097/PHM.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ilfeld BM, Grant SA, Gilmore CA, et al. Neurostimulation for postsurgical analgesia: a novel system enabling ultrasound‐guided percutaneous peripheral nerve stimulation. Pain Pract. 2017;17(7):892–901. doi: 10.1111/papr.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ilfeld BM, Finneran JJ, Gabriel RA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof-of-concept study. Reg Anesth Pain Med. 2019;44(3):310–318. doi: 10.1136/rapm-2018-100121 [DOI] [PubMed] [Google Scholar]
- 183.Ilfeld BM, Gabriel RA, Said ET, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg Anesth Pain Med. 2018;43(6):580–589. doi: 10.1097/AAP.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ilfeld BM, Plunkett A, Vijjeswarapu AM, et al. Percutaneous peripheral nerve stimulation (neuromodulation) for postoperative pain: a randomized, sham-controlled pilot study. Anesthesiology. 2021;135(1):95–110. doi: 10.1097/ALN.0000000000003776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gabriel RA, Swisher MW, Ilfeld BM. Percutaneous peripheral nerve stimulation for acute postoperative pain. Pain Manag. 2019;9(4):347–354. doi: 10.2217/pmt-2018-0094 [DOI] [PubMed] [Google Scholar]
- 186.Deer TR, Gilmore CA, Desai MJ, et al. Percutaneous peripheral nerve stimulation of the medial branch nerves for the treatment of chronic axial back pain in patients after radiofrequency ablation. Pain Med. 2021;22(3):548–560. doi: 10.1093/pm/pnaa432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gilmore CA, Kapural L, McGee MJ, Boggs JW. Percutaneous peripheral nerve stimulation for chronic low back pain: prospective case series with 1 year of sustained relief following short‐term implant. Pain Pract. 2020;20(3):310–320. doi: 10.1111/papr.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schoenen J, Jensen RH, Lanteri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–830. doi: 10.1177/0333102412473667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 2016;19(4):373–384. doi: 10.1111/ner.12438 [DOI] [PubMed] [Google Scholar]
- 190.Holsheimer J, Buitenweg JR, Das J, de Sutter P, Manola L, Nuttin B. The effect of pulse width and contact configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery. 2011;68(5):1452–1461. discussion 1461. doi: 10.1227/NEU.0b013e31820b4f47 [DOI] [PubMed] [Google Scholar]
- 191.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13(3):99–104. doi: 10.1016/0166-2236(90)90185-d [DOI] [PubMed] [Google Scholar]
- 192.Kilgore KL, Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation. 2014;17(3):242–254. discussion 254-5. doi: 10.1111/ner.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Isagulyan E, Tkachenko V, Semenov D, et al. The effectiveness of various types of electrical stimulation of the spinal cord for chronic pain in patients with postherpetic neuralgia: a literature review. Pain Res Manag. 2023;2023:6015680. doi: 10.1155/2023/6015680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Strand NH, D’Souza R, Wie C, et al. Mechanism of action of peripheral nerve stimulation. Curr Pain Headache Rep. 2021;25(7):47. doi: 10.1007/s11916-021-00962-3 [DOI] [PubMed] [Google Scholar]
- 195.Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep. 2012;16(3):217–225. doi: 10.1007/s11916-012-0260-4 [DOI] [PubMed] [Google Scholar]
- 196.Barchini J, Tchachaghian S, Shamaa F, et al. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 2012;215:196–208. doi: 10.1016/j.neuroscience.2012.04.057 [DOI] [PubMed] [Google Scholar]
- 197.Sato KL, King EW, Johanek LM, Sluka KA. Spinal cord stimulation reduces hypersensitivity through activation of opioid receptors in a frequency-dependent manner. Eur J Pain. 2013;17(4):551–561. doi: 10.1002/j.1532-2149.2012.00220.x [DOI] [PubMed] [Google Scholar]
- 198.Gao J, Wu M, Li L, et al. Effects of spinal cord stimulation with “standard clinical” and higher frequencies on peripheral blood flow in rats. Brain Res. 2010;1313:53–61. doi: 10.1016/j.brainres.2009.11.072 [DOI] [PubMed] [Google Scholar]
- 199.Lee KY, Bae C, Lee D, et al. Low-intensity, Kilohertz frequency spinal cord stimulation differently affects excitatory and inhibitory neurons in the rodent superficial dorsal horn. Neuroscience. 2020;428:132–139. doi: 10.1016/j.neuroscience.2019.12.031 [DOI] [PubMed] [Google Scholar]
- 200.D’Souza RS, Her YF. Stimulation holiday rescues analgesia after habituation and loss of efficacy from 10-kilohertz dorsal column spinal cord stimulation. Reg Anesth Pain Med. 2022;47(12):722–727. doi: 10.1136/rapm-2022-103881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hagedorn JM, Romero J, Thuc Ha C, Bendel MA, D’Souza RS. Paresthesia-based versus high-frequency spinal cord stimulation: a retrospective, real-world, single-center comparison. Neuromodulation. 2021. doi: 10.1111/ner.13497 [DOI] [PubMed] [Google Scholar]
- 202.Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 2017;20(6):525–533. doi: 10.1111/ner.12624 [DOI] [PubMed] [Google Scholar]
- 203.Crosby ND, Janik JJ, Grill WM. Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation. J Neurophysiol. 2017;117(1):136–147. doi: 10.1152/jn.00701.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.D’Souza RS, Strand N. Neuromodulation with burst and tonic stimulation decreases opioid consumption: a post hoc analysis of the Success Using Neuromodulation with BURST (SUNBURST) randomized controlled trial. Neuromodulation. 2021;24(1):135–141. doi: 10.1111/ner.13273 [DOI] [PubMed] [Google Scholar]
- 205.De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation. 2016;19(1):47–59. doi: 10.1111/ner.12368 [DOI] [PubMed] [Google Scholar]
- 206.Chakravarthy K, Fishman MA, Zuidema X, Hunter CW, Levy R. Mechanism of action in burst spinal cord stimulation: review and recent advances. Pain Med. 2019;20(Suppl 1):S13–S22. doi: 10.1093/pm/pnz073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dostrovsky JO, Millar J, Wall PD. The immediate shift of afferent drive to dorsal column nucleus cells following deafferentation: a comparison of acute and chronic deafferentation in gracile nucleus and spinal cord. Exp Neurol. 1976;52(3):480–495. doi: 10.1016/0014-4886(76)90219-3 [DOI] [PubMed] [Google Scholar]
- 208.Lempka SF, Patil PG. Innovations in spinal cord stimulation for pain. Curr Opin Biomed Eng. 2018;8:51–60. doi: 10.1016/j.cobme.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–134. doi: 10.1016/S1474-4422(19)30414-4 [DOI] [PubMed] [Google Scholar]
- 210.Parker J, Karantonis D, Single P. Hypothesis for the mechanism of action of ECAP-controlled closed-loop systems for spinal cord stimulation. Healthc Technol Lett. 2020;7(3):76–80. doi: 10.1049/htl.2019.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vallejo R, Kelley CA, Gupta A, Smith WJ, Vallejo A, Cedeño DL. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol Pain. 2020;16:1744806920918057. doi: 10.1177/1744806920918057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gritsch S, Lu J, Thilemann S, et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat Commun. 2014;5:5472. doi: 10.1038/ncomms6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tilley DM, Cedeño DL, Vetri F, Platt DC, Vallejo R. Differential target multiplexed spinal cord stimulation programming modulates proteins involved in ion regulation in an animal model of neuropathic pain. Mol Pain. 2022;18:17448069211060181. doi: 10.1177/17448069211060181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5–12. doi: 10.1152/jn.00457.2012 [DOI] [PubMed] [Google Scholar]
- 215.Gilbert JE, Titus N, Zhang T, Esteller R, Grill WM. Surround inhibition mediates pain relief by low amplitude spinal cord stimulation: modeling and measurement. eNeuro. 2022;9(5):ENEURO.0058–22.2022. doi: 10.1523/ENEURO.0058-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Paz-Solís J, Thomson S, Jain R, Chen L, Huertas I, Doan Q. Exploration of high- and low-frequency options for subperception spinal cord stimulation using neural dosing parameter relationships: the HALO study. Neuromodulation. 2022;25(1):94–102. doi: 10.1111/ner.13390 [DOI] [PubMed] [Google Scholar]
- 217.Kapural L, Patterson DG, Li S, et al. Multiphase spinal cord stimulation in participants with chronic back or leg pain: results of the BENEFIT-02 randomized clinical trial. Neuromodulation. 2023;26(7):1400–1411. doi: 10.1016/j.neurom.2023.05.006 [DOI] [PubMed] [Google Scholar]
- 218.Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;18(8):1048–1067. doi: 10.1111/papr.12692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Deer TR, Pope JE, Lamer TJ, et al. The neuromodulation appropriateness consensus committee on best practices for dorsal root ganglion stimulation. Neuromodulation. 2019;22(1):1–35. doi: 10.1111/ner.12845 [DOI] [PubMed] [Google Scholar]
- 220.Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20(Suppl 1):S23–S30. doi: 10.1093/pm/pnz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Amedee T, Ellie E, Dupouy B, Vincent JD. Voltage-dependent calcium and potassium channels in Schwann cells cultured from dorsal root ganglia of the mouse. J Physiol. 1991;441:35–56. doi: 10.1113/jphysiol.1991.sp018737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;82(Suppl 6):S27–S35. doi: 10.1016/S0304-3959(99)00135-9 [DOI] [PubMed] [Google Scholar]
- 223.Sheng SR, Wang XY, Xu HZ, Zhu GQ, Zhou YF. Anatomy of large animal spines and its comparison to the human spine: a systematic review. Eur Spine J. 2010;19(1):46–56. doi: 10.1007/s00586-009-1192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hao H, Ramli R, Wang C, et al. Dorsal root ganglia control nociceptive input to the central nervous system. PLoS Biol. 2023;21(1):e3001958. doi: 10.1371/journal.pbio.3001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103(2):360–376. doi: 10.1097/00000542-200508000-00020 [DOI] [PubMed] [Google Scholar]
- 226.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18(1):24–32. discussion 32. doi: 10.1111/ner.12247 [DOI] [PubMed] [Google Scholar]
- 227.Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur J Pain. 2004;8(2):135–143. doi: 10.1016/S1090-3801(03)00086-7 [DOI] [PubMed] [Google Scholar]
- 228.Estivill-Torrus G, Martinez-Padilla AB, Sanchez-Salido L, Evercooren AB, Garcia-Diaz B. The dorsal root ganglion as a target for neurorestoration in neuropathic pain. Neural Regen Res. 2024;19(2):296–301. doi: 10.4103/1673-5374.374655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.D’Souza RS, Kubrova E, Her YF, et al. Dorsal root ganglion stimulation for lower extremity neuropathic pain syndromes: an evidence-based literature review. Adv Ther. 2022;39(10):4440–4473. doi: 10.1007/s12325-022-02244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15(10):1669–1685. doi: 10.1111/pme.12413 [DOI] [PubMed] [Google Scholar]
- 231.Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681. doi: 10.1097/j.pain.0000000000000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chapman KB, Van Roosendaal BW, Van Helmond N, Yousef TA. Unilateral dorsal root ganglion stimulation lead placement with resolution of bilateral lower extremity symptoms in diabetic peripheral neuropathy. Cureus. 2020;12(9):e10735. doi: 10.7759/cureus.10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cesmebasi A. Chapter 33–Anatomy of the dorsal root ganglion. In: Tubbs RS, Rizk EB, Shoja MM, Loukas M, Barbaro N, Spinner RJ, editors. Nerves and Nerve Injuries. Academic Press; 2015:471–476. [Google Scholar]
- 234.London D, Mogilner A. Spinal cord stimulation: new waveforms and technology. Neurosurg Clin N Am. 2022;33(3):287–295. doi: 10.1016/j.nec.2022.02.006 [DOI] [PubMed] [Google Scholar]
- 235.Deer TR, Levy RM, Kramer J, et al. Comparison of paresthesia coverage of patient’s pain: dorsal root ganglion vs spinal cord stimulation. An ACCURATE study sub-analysis. Neuromodulation. 2019;22(8):930–936. doi: 10.1111/ner.12920 [DOI] [PubMed] [Google Scholar]
- 236.Mekhail N, Deer TR, Kramer J, et al. Paresthesia-free dorsal root ganglion stimulation: an ACCURATE study sub-analysis. Neuromodulation. 2020;23(2):185–195. doi: 10.1111/ner.12942 [DOI] [PubMed] [Google Scholar]
- 237.Patterson DG, Mehta P, Baksh J, et al. Thoracic and lumbar dorsal root ganglion spinal stimulation. In: Deer TR, Pope JE, Lamer TJ, Provenzano D, editors. Deer’s Treatment of Pain: An Illustrated Guide for Practitioners. Springer International Publishing; 2019:581–587. [Google Scholar]
- 238.Hunter CW, Patel D. Percutaneous sacral nerve stimulation. In: Deer TR, Pope JE, Lamer TJ, Provenzano D, editors. Deer’s Treatment of Pain: An Illustrated Guide for Practitioners. Springer International Publishing; 2019:589–598. [Google Scholar]
- 239.Deer TR, Pope JE. Dorsal root ganglion stimulation approval by the food and drug administration: advice on evolving the process. Expert Rev Neurother. 2016;16(10):1123–1125. doi: 10.1080/14737175.2016.1206817 [DOI] [PubMed] [Google Scholar]
- 240.Rowe J, Deer TR, Mehta P, et al. Sacral Dorsal Root Ganglion Spinal Stimulation. In: Deer TR, Pope JE, Lamer TJ, Provenzano D, editors. Deer’s Treatment of Pain: An Illustrated Guide for Practitioners. Springer International Publishing; 2019:599–605. [Google Scholar]
- 241.Skaribas IM, Peccora C, Skaribas E. Single S1 dorsal root ganglia stimulation for intractable complex regional pain syndrome foot pain after lumbar spine surgery: a case series. Neuromodulation. 2019;22(1):101–107. doi: 10.1111/ner.12780 [DOI] [PubMed] [Google Scholar]
- 242.Shen J, Wang HY, Chen JY, Liang BL. Morphologic analysis of normal human lumbar dorsal root ganglion by 3D MR imaging. AJNR Am J Neuroradiol. 2006;27(10):2098–2103. [PMC free article] [PubMed] [Google Scholar]
- 243.Hasegawa T, Mikawa Y, Watanabe R, An HS. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine. 1996;21(9):1005–1009. doi: 10.1097/00007632-199605010-00001 [DOI] [PubMed] [Google Scholar]
- 244.Arslan M, Comert A, Acar HI, et al. Nerve root to lumbar disc relationships at the intervertebral foramen from a surgical viewpoint: an anatomical study. Clin Anat. 2012;25(2):218–223. doi: 10.1002/ca.21213 [DOI] [PubMed] [Google Scholar]
- 245.Dubovy P, Klusakova I, Svizenska I, Brazda V. Satellite glial cells express IL-6 and corresponding signal-transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron Glia Biol. 2010;6(1):73–83. doi: 10.1017/S1740925X10000074 [DOI] [PubMed] [Google Scholar]
- 246.Mitterreiter JG, Ouwendijk WJD, van Velzen M, van Nierop GP, Osterhaus A, Verjans G. Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors. Eur J Immunol. 2017;47(7):1181–1187. doi: 10.1002/eji.201746989 [DOI] [PubMed] [Google Scholar]
- 247.Galvin DA, M C. The role of T-lymphocytes in neuropathic pain initiation, development of chronicity and treatment. Brain Behav Immun Health. 2021;18:100371. doi: 10.1016/j.bbih.2021.100371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ferrara V, Toti A, Ghelardini C, Di Cesare Mannelli L. Interferon-gamma and neuropathy: balance between pain and neuroprotection. Neural Regen Res. 2022;17(12):2700–2701. doi: 10.4103/1673-5374.339484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yuan H, Du S, Chen L, Xu X, Wang Y, Ji F. Hypomethylation of nerve growth factor (NGF) promotes binding of C/EBPalpha and contributes to inflammatory hyperalgesia in rats. J Neuroinflammation. 2020;17(1):34. doi: 10.1186/s12974-020-1711-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cragg JJ, Scott AL, Ramer MS. Depletion of spinal 5-HT accelerates mechanosensory recovery in the deafferented rat spinal cord. Exp Neurol. 2010;222(2):277–284. doi: 10.1016/j.expneurol.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 251.Theile JW, Cummins TR. Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes. Front Pharmacol. 2011;2:54. doi: 10.3389/fphar.2011.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu X, Chung K, Chung JM. Ectopic discharges and adrenergic sensitivity of sensory neurons after spinal nerve injury. Brain Res. 1999;849(1–2):244–247. doi: 10.1016/s0006-8993(99)02165-4 [DOI] [PubMed] [Google Scholar]
- 253.Mendell LM. The path to discovery of windup and central sensitization. Front Pain Res. 2022;3:833104. doi: 10.3389/fpain.2022.833104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kent AR, Min X, Hogan QH, Kramer JM. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation. 2018;21(3):234–246. doi: 10.1111/ner.12754 [DOI] [PubMed] [Google Scholar]
- 255.Huygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2-L3 dorsal root ganglia induces effective pain relief in the low back. Pain Pract. 2018;18(2):205–213. doi: 10.1111/papr.12591 [DOI] [PubMed] [Google Scholar]
- 256.Bergman RA, Bergman RA. Compendium of Human Anatomic Variation: Text, Atlas, and World Literature. Lippincott Williams & Wilkins; 1988. [Google Scholar]
- 257.Piedade GS, Vesper J, Chatzikalfas A, Slotty PJ. Cervical and high-thoracic dorsal root ganglion stimulation in chronic neuropathic pain. Neuromodulation. 2019;22(8):951–955. doi: 10.1111/ner.12916 [DOI] [PubMed] [Google Scholar]
- 258.Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019;22(1):61–79. doi: 10.1111/ner.12796 [DOI] [PubMed] [Google Scholar]
- 259.Anthony CL, Tora MS, Bentley JN, Texakalidis P, Boulis NM. Dorsal root ganglion stimulation for thoracic neuralgia: a report of six cases. Cureus. 2019;11(5):e4615. doi: 10.7759/cureus.4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pope JE, Provenzano D, McRoberts P, Deer T. The proper use of neurostimulation for hand pain. Hand Clin. 2016;32(1):81–90. doi: 10.1016/j.hcl.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 261.Hoffman HH. An analysis of the sympathetic trunk and rami in the cervical and upper thoracic regions in man. Ann Surg. 1957;145(1):94–103. doi: 10.1097/00000658-195701000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hoffman HH, Jacobs MW, Kuntz A. Nerve fiber components of communicating rami and sympathetic roots in man. Anat Rec. 1956;126(1):29–41. doi: 10.1002/ar.1091260104 [DOI] [PubMed] [Google Scholar]
- 263.Yabuki S, Kikuchi S. Positions of dorsal root ganglia in the cervical spine. An anatomic and clinical study. Spine. 1996;21(13):1513–1517. doi: 10.1097/00007632-199607010-00004 [DOI] [PubMed] [Google Scholar]
- 264.Deer TR, Russo M, Grider JS, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations on best practices for cervical neurostimulation. Neuromodulation. 2022;25(1):35–52. doi: 10.1016/j.neurom.2021.10.013 [DOI] [PubMed] [Google Scholar]
- 265.Hogan QH. Epidural anatomy examined by cryomicrotome section. Influence of age, vertebral level, and disease. Reg Anesth. 1996;21(5):395–406. [PubMed] [Google Scholar]
- 266.Ushiku C, Soshi S, Inoue T, et al. The position of the vertebral artery V1 segment relative to the C7 vertebra. J Orthop Sci. 2021;26(2):203–206. doi: 10.1016/j.jos.2020.03.020 [DOI] [PubMed] [Google Scholar]
- 267.Lirk P, Kolbitsch C, Putz G, et al. Cervical and high thoracic ligamentum flavum frequently fails to fuse in the midline. Anesthesiology. 2003;99(6):1387–1390. doi: 10.1097/00000542-200312000-00023 [DOI] [PubMed] [Google Scholar]
- 268.Chapman KB, Yousef TA, Foster A, M DS-H, van Helmond N. Mechanisms for the clinical utility of low-frequency stimulation in neuromodulation of the dorsal root ganglion. Neuromodulation. 2021;24(4):738–745. doi: 10.1111/ner.13323 [DOI] [PubMed] [Google Scholar]
- 269.Joosten EA, Franken G. Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain. 2020;161(Suppl 1):S104–S113. doi: 10.1097/j.pain.0000000000001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chapman KB, Groenen PS, Vissers KC, van Helmond N, Stanton-Hicks MD. The pathways and processes underlying spinal transmission of low back pain: observations from dorsal root ganglion stimulation treatment. Neuromodulation. 2021;24(4):610–621. doi: 10.1111/ner.13150 [DOI] [PubMed] [Google Scholar]
- 271.Loken LS, Duff EP, Tracey I. Low-threshold mechanoreceptors play a frequency-dependent dual role in subjective ratings of mechanical allodynia. J Neurophysiol. 2017;118(6):3360–3369. doi: 10.1152/jn.00977.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ikeda H, Asai T, Randic M, Murase K. Robust suppression of afferent-induced excitation in the rat spinal dorsal horn after conditioning low-frequency stimulation. J Neurophysiol. 1999;82(4):1957–1964. doi: 10.1152/jn.1999.82.4.1957 [DOI] [PubMed] [Google Scholar]
- 273.Bardoni R, Tawfik VL, Wang D, et al. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 2014;81(6):1312–1327. doi: 10.1016/j.neuron.2014.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chapman KB, Yousef TA, Vissers KC, van Helmond N, M DS-H. Very low frequencies maintain pain relief from dorsal root ganglion stimulation: an evaluation of dorsal root ganglion neurostimulation frequency tapering. Neuromodulation. 2021;24(4):746–752. doi: 10.1111/ner.13322 [DOI] [PubMed] [Google Scholar]
- 275.Arle JE. Chapter 6–Waveforms and mechanisms in neuromodulation. In: Arle JE, Shils JL, editors. Essential Neuromodulation (Second Edition). Academic Press; 2022:119–130. [Google Scholar]
- 276.Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: burst vs conventional stimulation paradigm. Neuromodulation. 2019;22(8):943–950. doi: 10.1111/ner.12908 [DOI] [PMC free article] [PubMed] [Google Scholar]