Abstract
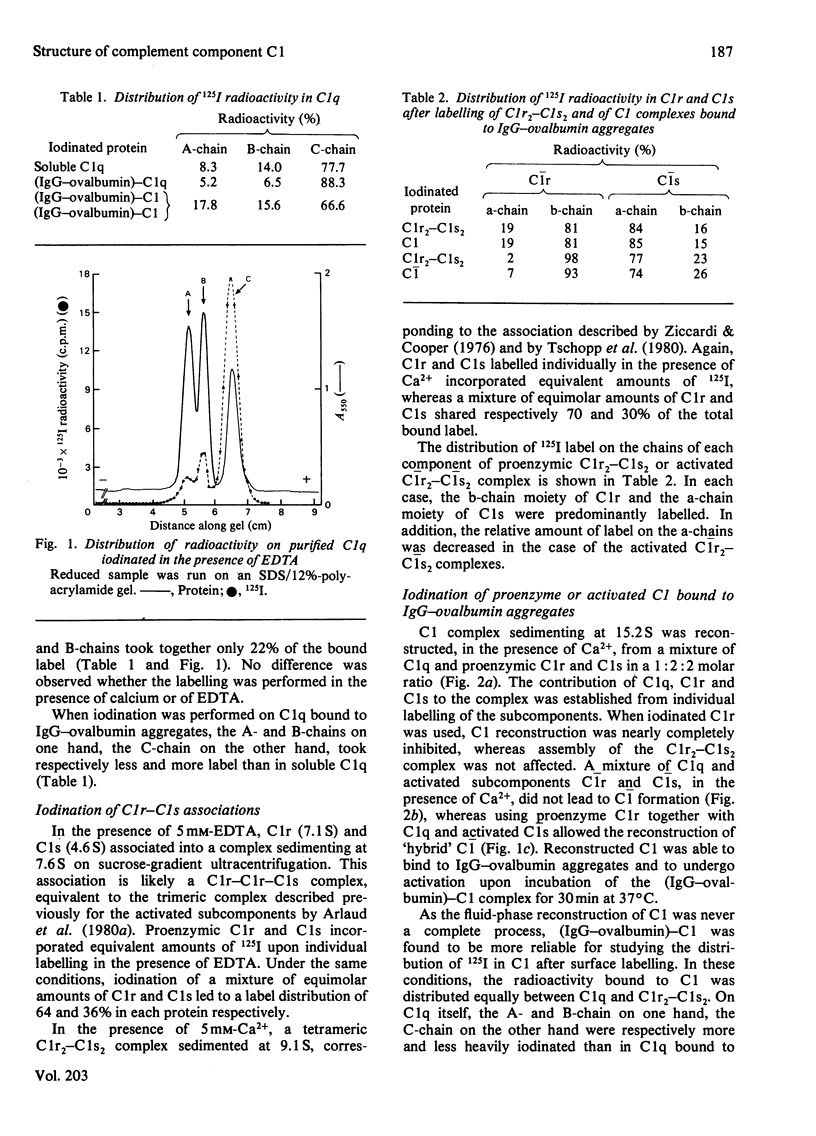
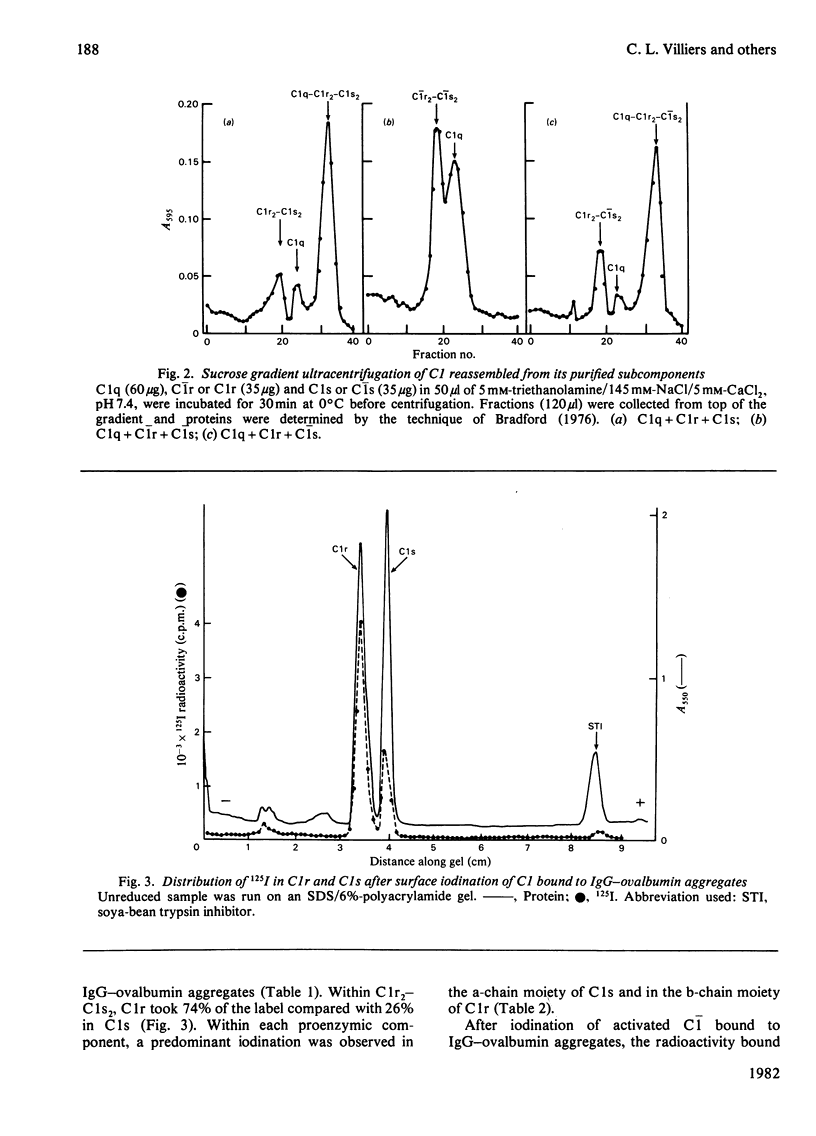
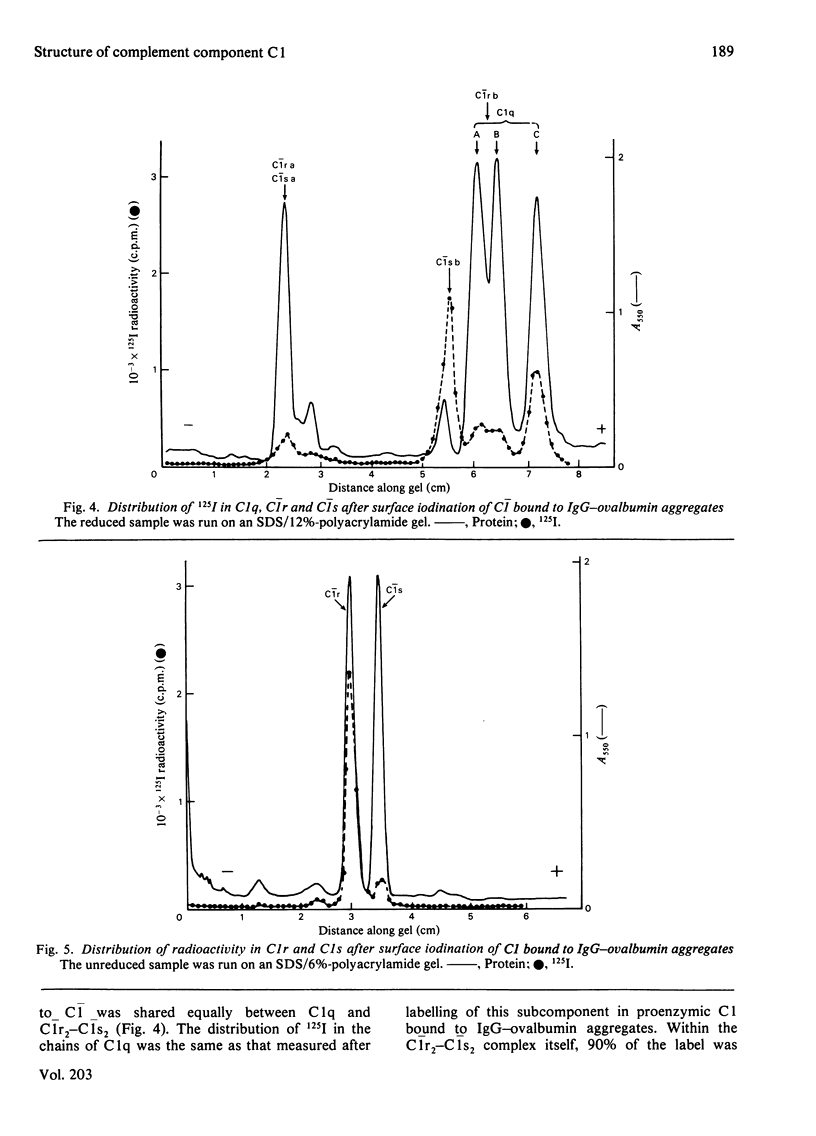
Lactoperoxidase-catalysed surface iodination and sucrose-gradient ultracentrifugation were used to investigate the structure of human complement component C1. 1. Proenzymic subcomponents C1r and C1s associated to form a trimeric C1r2-C1s complex (7.6 S) in the presence of EDTA, and a tetrameric Clr2-C1s2 complex (9.1 S) in the presence of Ca2+. Iodination of the 9.1 S complex led to a predominant labelling of C1r (70%) over C1s (30%), essentially located in the b-chain moiety of C1r and in the a-chain moiety of C1s. 2. Reconstruction of proenzymic soluble C1 (15.2 S) from C1q, C1r and C1s was partially inhibited when C1s labelled in its monomeric form was used and almost abolished when iodinated C1r was used. Reconstruction of fully activated C1 was not possible, whereas hybrid C1q-C1r2-C1s2 complex was obtained. 3. Iodination of proenzymic or activated C1 bound to IgG-ovalbumin aggregates led to an equal distribution of the radioactivity between C1q and C1r2-C1s2. With regard to C1q, the label distribution between the three chains was similar whether C1 was in its proenzymic or activated form. Label distribution in the C1r2-C1s2 moiety of C1 was the same as that obtained for isolated C1r2-C1s2, and this was also true for the corresponding activated components. However, two different labelling patterns were found, corresponding to the proenzyme and the activated states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Chesne S., Villiers C. L., Colomb M. G. A study on the structure and interactions of the C1 sub-components C1r and C1s in the fluid phase. Biochim Biophys Acta. 1980 Nov 6;616(1):105–115. doi: 10.1016/0005-2744(80)90268-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Villiers C. L., Chesne S., Colomb M. G. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980 Nov 6;616(1):116–129. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Assimeh S. N., Painter R. H. The macromolecular structure of the first component of complement. J Immunol. 1975 Aug;115(2):488–494. [PubMed] [Google Scholar]
- Bauer J., Valet G. Conformational changes of the subunits C1q, C1r and C1s of human complement component C1 demonstrated by 125I labeling. Biochim Biophys Acta. 1981 Aug 28;670(1):129–133. doi: 10.1016/0005-2795(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chesne S., Villiers C. L., Arlaud G. J., Lacroix M. B., Colomb M. G. Fluid-phase interaction of C1 inhibitor (C1 Inh) and the subcomponents C1r and C1s of the first component of complement, C1. Biochem J. 1982 Jan 1;201(1):61–70. doi: 10.1042/bj2010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Folkerd E. J., Gardner B., Hughes-Jones N. C. The relationship between the binding ability and the rate of activation of the complement component C1. Immunology. 1980 Sep;41(1):179–185. [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser C., Boesman M., Nordin J. H., Isliker H. Effect of chemical and enzymatic radioiodination on in vitro human Clq activities. J Immunol. 1973 Mar;110(3):820–828. [PubMed] [Google Scholar]
- Lin T. Y., Fletcher D. S. Interaction of human Clq with insoluble immunoglobulin aggregates. Immunochemistry. 1978 Feb;15(2):107–117. doi: 10.1016/0161-5890(78)90050-0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nagasawa S., Takahashi K., Koyama J. Isolation of a complex of the subcomponents of the activated first component of complement, Clr--Cls, from ACD-human plasma. FEBS Lett. 1974 May 1;41(2):280–282. doi: 10.1016/0014-5793(74)81229-9. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R. Isolation and comparison of the proenzyme and activated forms of the human serum complement subcomponents C1r and C1s. Biochem Soc Trans. 1976;4(1):127–129. doi: 10.1042/bst0040127. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nagasawa S., Koyama J. A gross structure of an activated form of a subunit of the first component of human complement. Clr. FEBS Lett. 1975 Jul 15;55(1):156–160. doi: 10.1016/0014-5793(75)80982-3. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Tschopp J., Villiger W., Fuchs H., Kilchherr E., Engel J. Assembly of subcomponents C1r and C1s of first component of complement: electron microscopic and ultracentrifugal studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7014–7018. doi: 10.1073/pnas.77.12.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1r subunit of the first complement component. J Immunol. 1974 May;112(5):1667–1673. [PubMed] [Google Scholar]
- Villiers C. L., Duplaa A. M., Arlaud G. J., Colomb M. G. Fluid phase activation of proenzymic C1r purified by affinity chromatography. Biochim Biophys Acta. 1982 Jan 4;700(1):118–126. doi: 10.1016/0167-4838(82)90299-0. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]


