Abstract
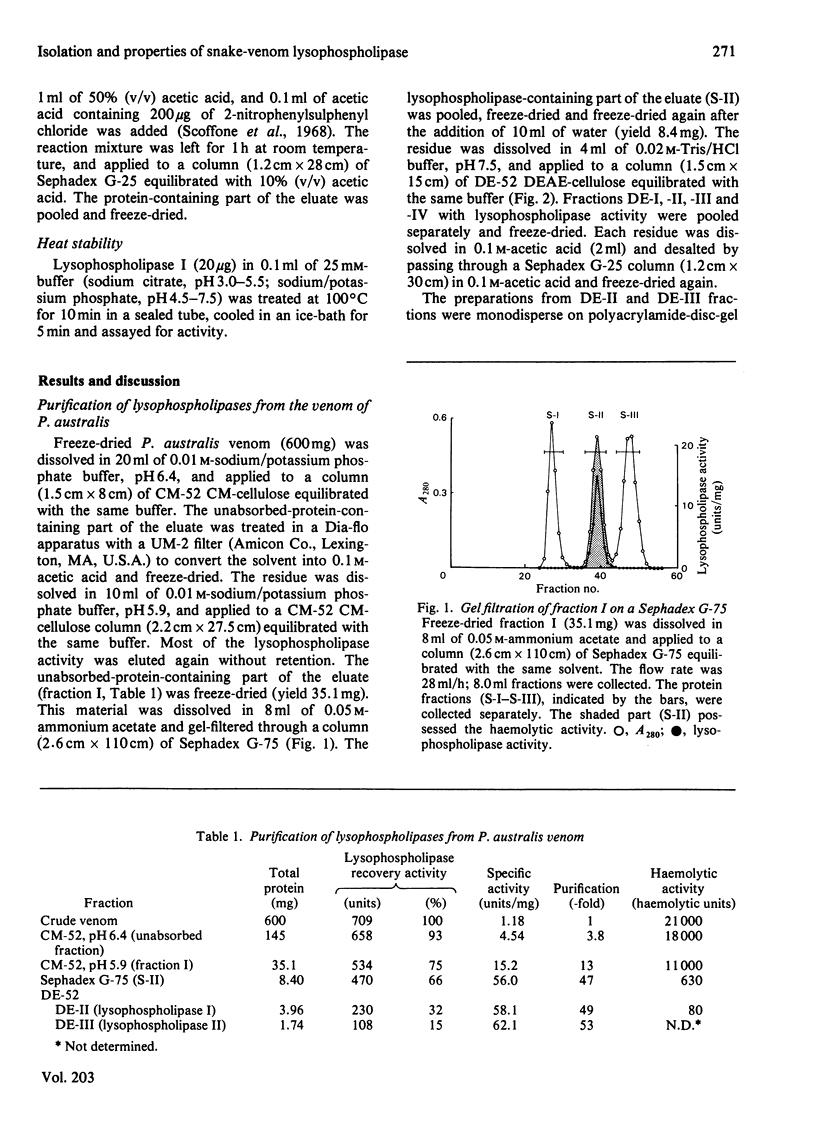
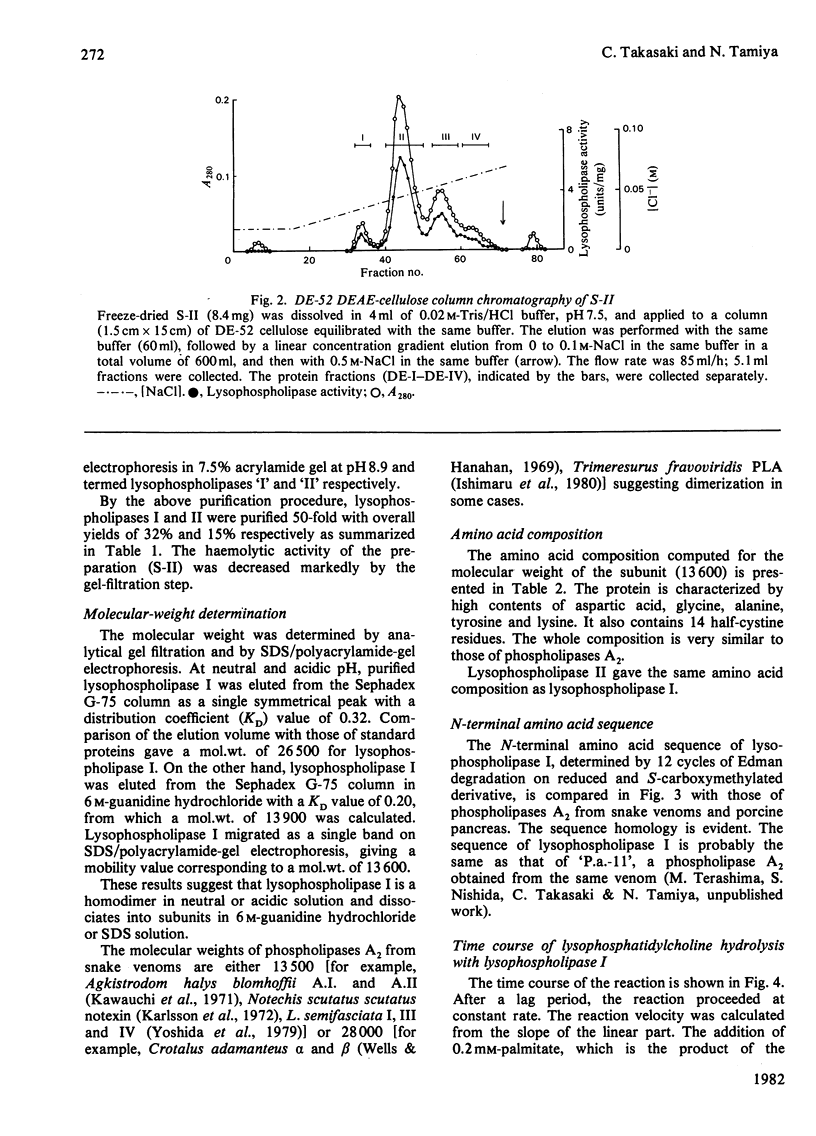
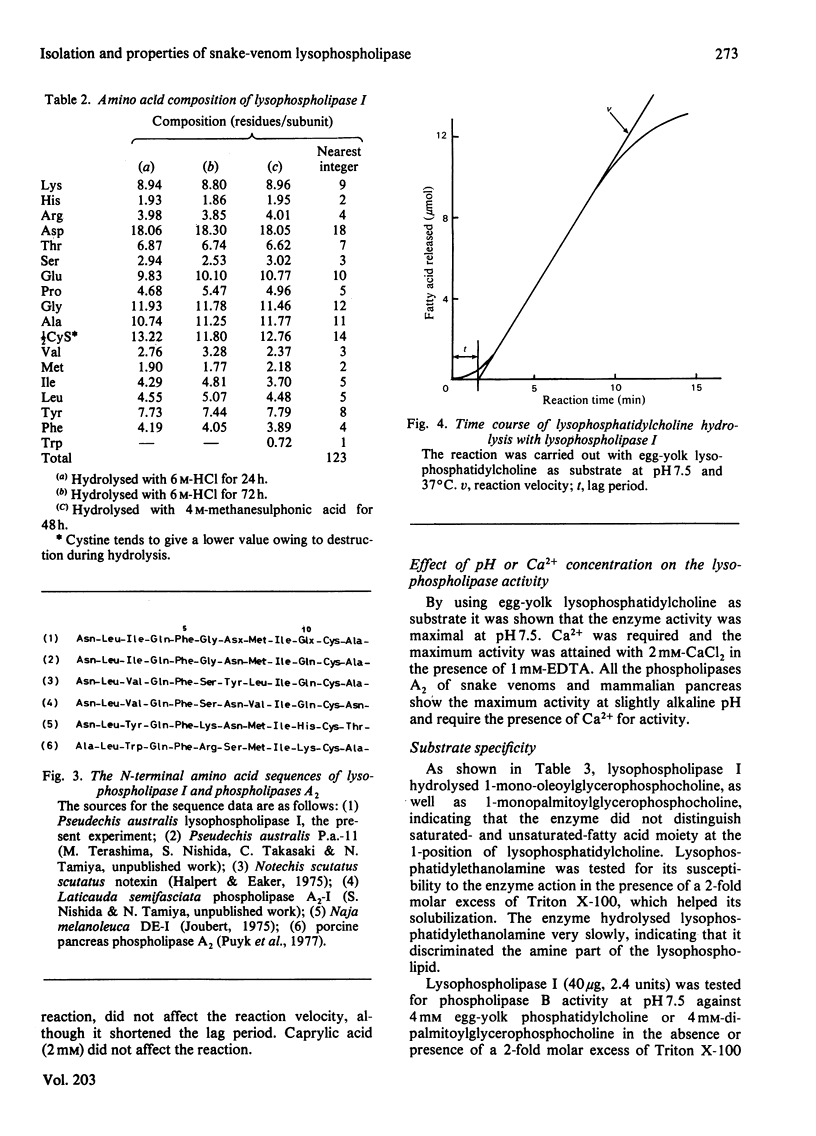
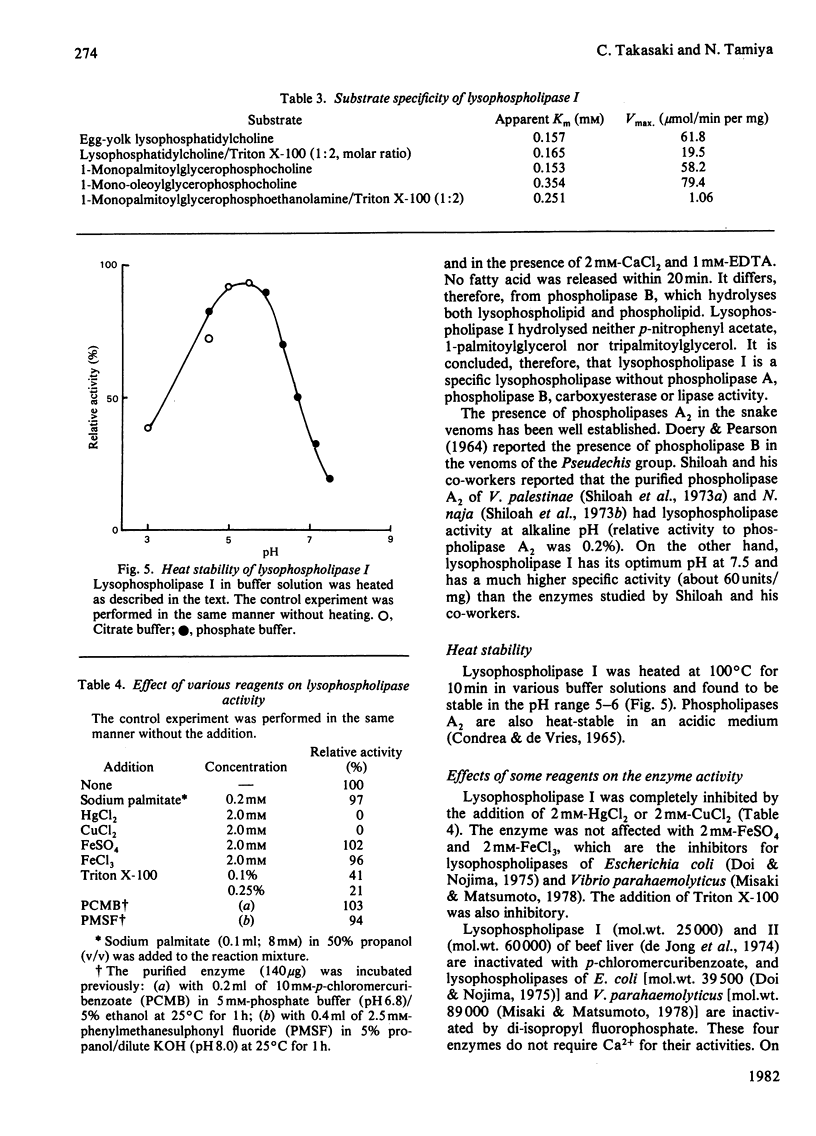
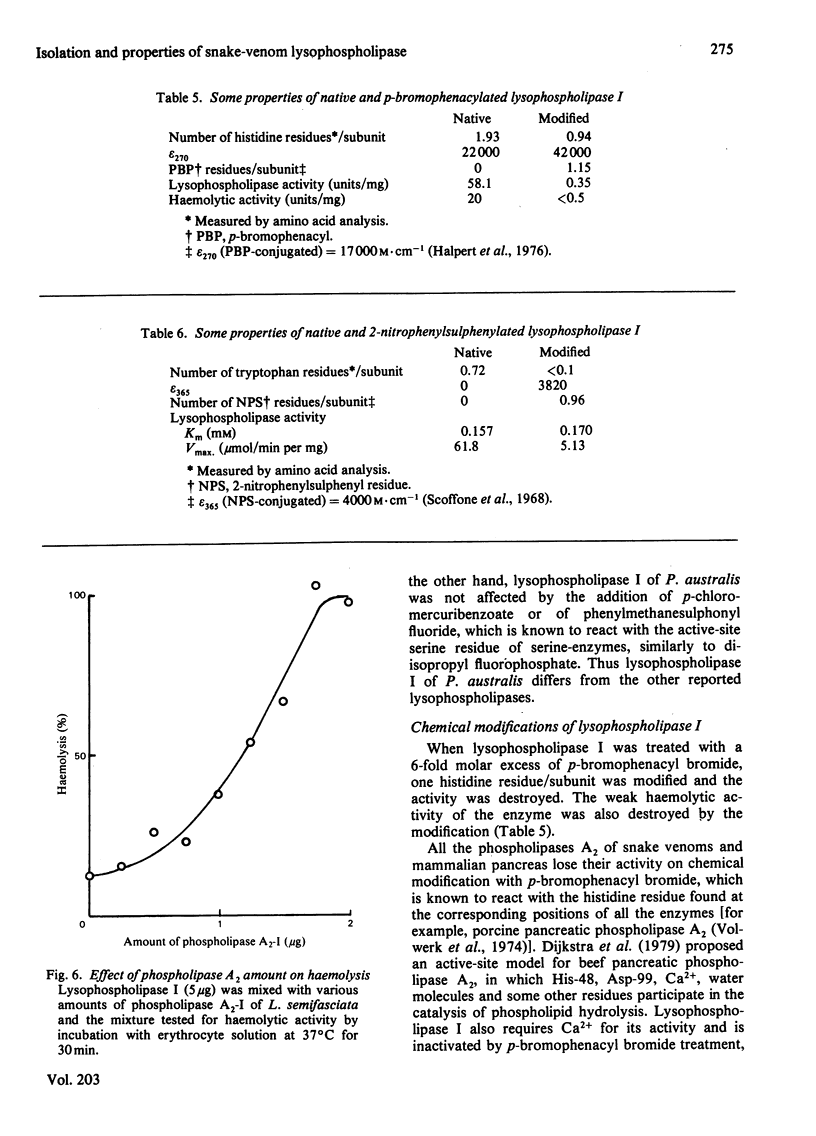
Two lysophospholipases were isolated from the venom of an Australian elapid snake (subfamily Acanthophiinae), Pseudechis australis, by sequential chromatography on CM-52 cellulose, Sephadex G-75 and DE-52 cellulose columns. They were very similar to each other. One of them, lysophospholipase I, was obtained as a homodimer, the monomer of which consisted of 123 amino acid residues with seven disulphide bridges. The amino acid composition and the N-terminal amino acid sequence of the enzyme were similar to those of phospholipase A2, Ca2+ was required for its activity and the maximum activity was attained at 2 mM-CaCl2 in the presence of 1 mM-EDTA. The optimum pH was 7.5. Lysophospholipase I hydrolysed lysophosphatidylcholine more rapidly than lysophosphatidylethanolamine. It did not hydrolyse, however, phosphatidylcholine, 1-palmitoylglycerol, tripalmitoylglycerol or p-nitrophenyl acetate. Modification of the enzyme with p-bromophenacyl bromide or 2-nitrophenylsulphenyl chloride suppressed the activity. A strong direct haemolytic activity was exhibited when the lysophospholipase was present together with phospholipase A2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condrea E. Membrane-active polypeptides from snake venom: cardiotoxins and haemocytotoxins. Experientia. 1974 Feb 15;30(2):121–129. doi: 10.1007/BF01927688. [DOI] [PubMed] [Google Scholar]
- DOERY H. M., PEARSON J. E. Haemolysins in venoms of Australian snakes. Observations on the haemolysins of the venoms of some Australian snakes and the separation of phospholipase A from the venom of Pseudechis porphyriacus. Biochem J. 1961 Apr;78:820–827. doi: 10.1042/bj0780820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doery H. M., Pearson J. E. Phospholipase B in snake venoms and bee venom. Biochem J. 1964 Sep;92(3):599–602. doi: 10.1042/bj0920599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi O., Nojima S. Lysophospholipase of Escherichia coli. J Biol Chem. 1975 Jul 10;250(13):5208–5214. [PubMed] [Google Scholar]
- Halpert J., Eaker D. Amino acid sequence of a presynaptic neurotoxin from the venom of Notechis scutatus scutatus (Australian tiger snake). J Biol Chem. 1975 Sep 10;250(17):6990–6997. [PubMed] [Google Scholar]
- Halpert J., Eaker D., Karlsson E. The role of phospholipase activity in the action of a presynaptic neurotoxin from the venom of Notechis scutatus scutatus (Australian tiger snake). FEBS Lett. 1976 Jan 1;61(1):72–76. doi: 10.1016/0014-5793(76)80174-3. [DOI] [PubMed] [Google Scholar]
- Ishimaru K., Kihara H., Ohno M. Purification and properties of phospholipase A from venom of Trimeresurus flavoviridis (Habu snake). J Biochem. 1980 Aug;88(2):443–451. doi: 10.1093/oxfordjournals.jbchem.a132991. [DOI] [PubMed] [Google Scholar]
- Iwanaga S., Wallén P., Gröndahl N. J., Henschen A., Blombäck B. On the primary structure of human fibrinogen. Isolation and characterization of N-terminal fragments from plasmic digests. Eur J Biochem. 1969 Mar;8(2):189–199. doi: 10.1111/j.1432-1033.1969.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Joubert F. J. The amino acid sequence of phospholipase A, fractions DE-I and DE-II. Biochim Biophys Acta. 1975 Feb 27;379(2):345–359. [PubMed] [Google Scholar]
- Karlsson E., Eaker D., Rydén L. Purification of a presynaptic neurotoxin from the venom of the australian tiger snake Notechis scutatus scutatus. Toxicon. 1972 Jun;10(4):405–413. doi: 10.1016/0041-0101(72)90066-9. [DOI] [PubMed] [Google Scholar]
- Kawauchi S., Iwanaga S., Samejima Y., Suzuki T. Isolation and characterization of two phospholipase A's from the venom of Agkistrodon lays blomhoffii. Biochim Biophys Acta. 1971 Apr 27;236(1):142–160. doi: 10.1016/0005-2795(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Chemistry and pharmacology of polypeptide toxins in snake venoms. Annu Rev Pharmacol. 1972;12:265–286. doi: 10.1146/annurev.pa.12.040172.001405. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Misaki H., Matsumoto M. Purification of lysophospholipase of Vibrio parahaemolyticus and its properties. J Biochem. 1978 May;83(5):1395–1405. doi: 10.1093/oxfordjournals.jbchem.a132049. [DOI] [PubMed] [Google Scholar]
- PANGBORN M. C. A simplified purification of lecithin. J Biol Chem. 1951 Feb;188(2):471–476. [PubMed] [Google Scholar]
- Puijk W. C., Verheij H. M., De Haas G. H. The primary structure of phospholipase A2 from porcine pancreas. A reinvestigation. Biochim Biophys Acta. 1977 Jun 24;492(2):254–259. doi: 10.1016/0005-2795(77)90076-9. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Scoffone E., Fontana A., Rocchi R. Sulfenyl halides as modifying reagents for polypeptides and proteins. I. Modification of tryptophan residues. Biochemistry. 1968 Mar;7(3):971–979. doi: 10.1021/bi00843a014. [DOI] [PubMed] [Google Scholar]
- Shiloah J., Klibansky C., De Vries A. Phospholipase isoenzymes from Naja naja venom. II. Phospholipase A and B activities. Toxicon. 1973 Oct;11(6):491–497. doi: 10.1016/0041-0101(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Shiloah J., Klibansky C., de Vries A., Berger A. Phospholipase B activity of a purified phospholipase A from Vipera palestinae venom. J Lipid Res. 1973 May;14(3):267–278. [PubMed] [Google Scholar]
- Tamiya N., Arai H. Studies on sea-snake venoms. Crystallization of erabutoxins a and b from Laticauda semifasciata venom. Biochem J. 1966 Jun;99(3):624–630. doi: 10.1042/bj0990624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wells M. A., Hanahan D. J. Studies on phospholipase A. I. Isolation and characterization of two enzymes from Crotalus adamanteus venom. Biochemistry. 1969 Jan;8(1):414–424. doi: 10.1021/bi00829a057. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kudo T., Shinkai W., Tamiya N. Phospholipase A of sea snake Laticauda semifasciata venom. Isolation and properties of novel forms lacking tryptophan. J Biochem. 1979 Feb;85(2):379–388. doi: 10.1093/oxfordjournals.jbchem.a132344. [DOI] [PubMed] [Google Scholar]
- de Jong J. G., van den Bosch H., Rijken D., van Deenen L. L. Studies on lysophospholipases. 3. The complete purification of two proteins with lysophospholipase activity from beef liver. Biochim Biophys Acta. 1974 Oct 16;369(1):50–63. doi: 10.1016/0005-2760(74)90191-x. [DOI] [PubMed] [Google Scholar]


