Abstract
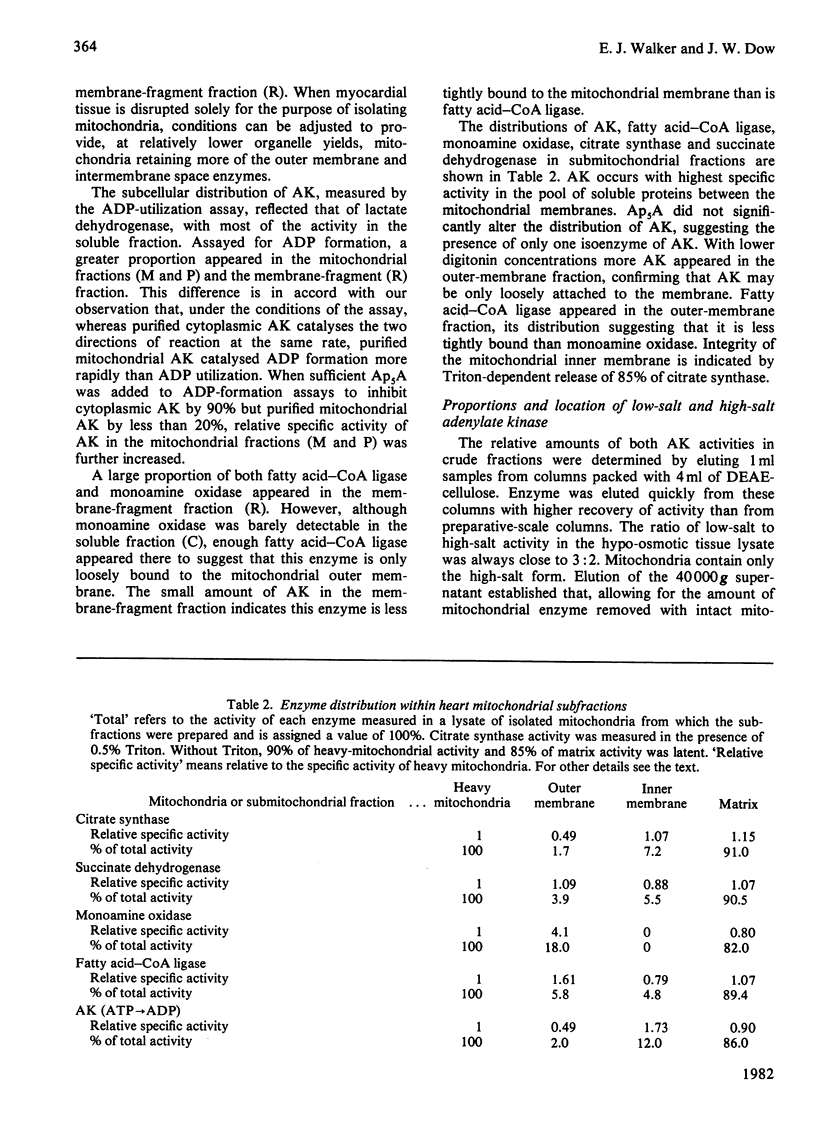
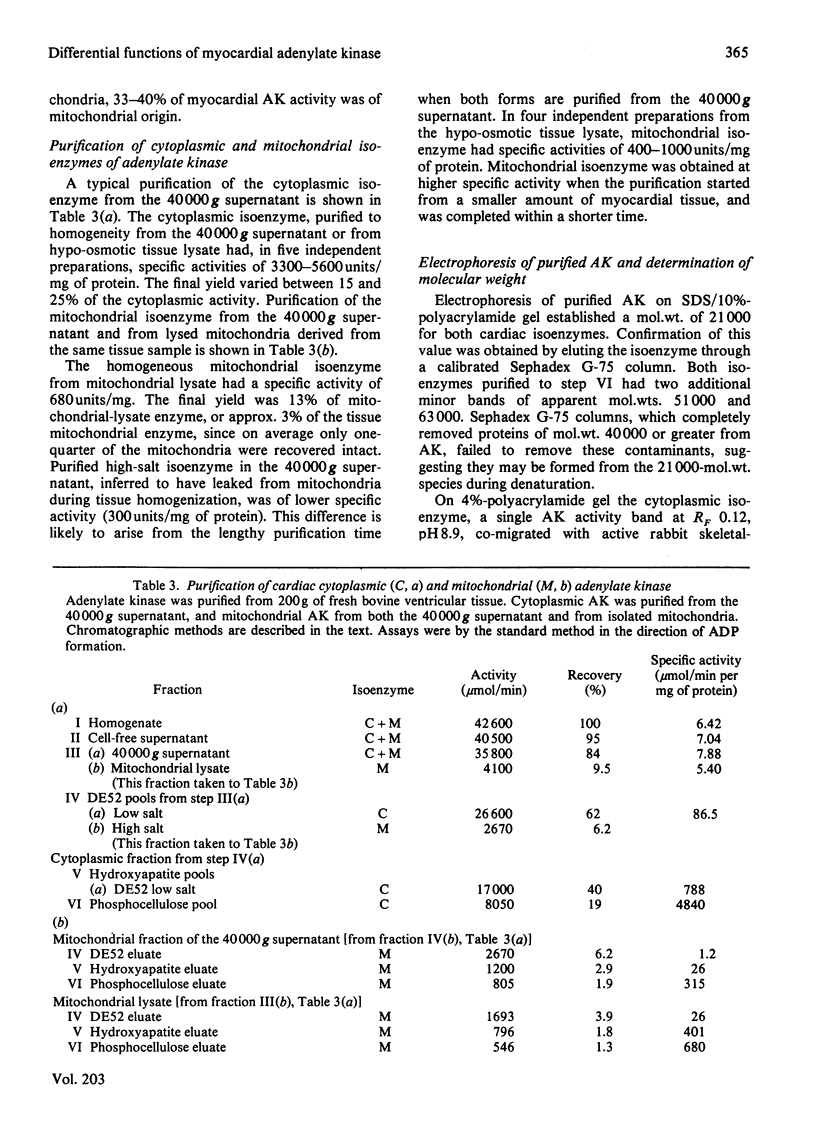
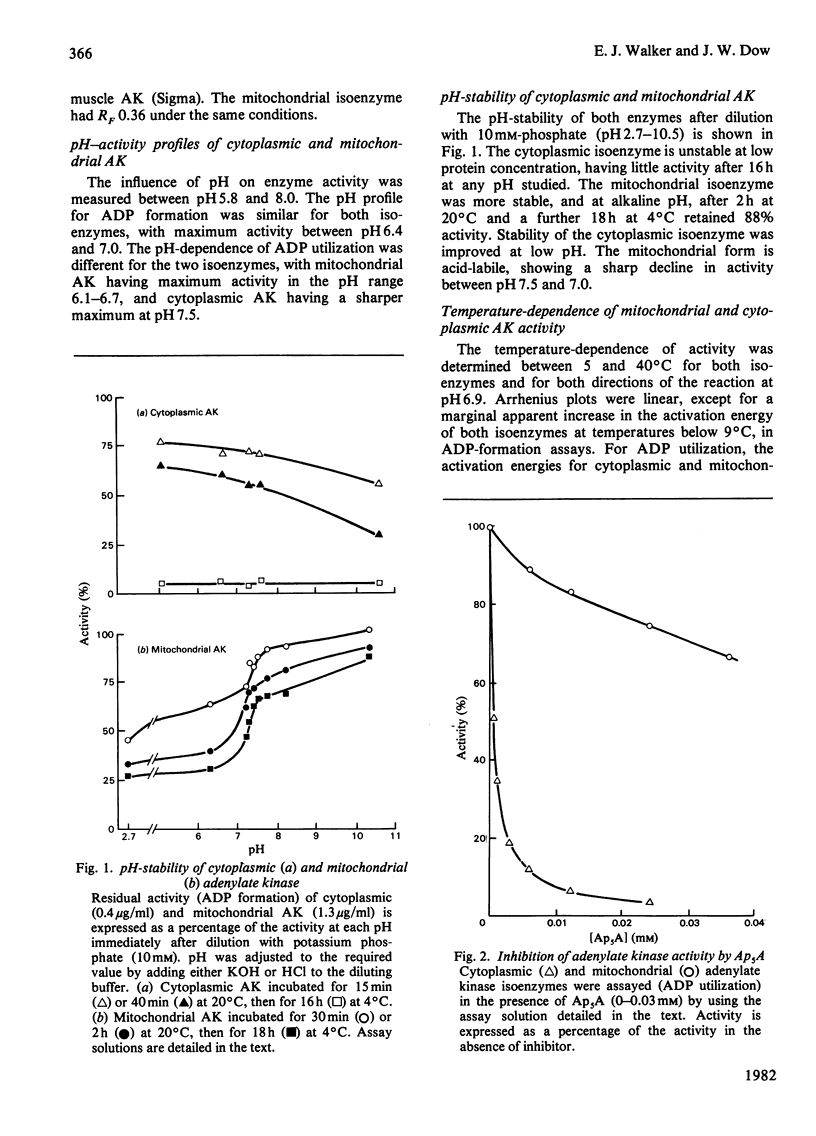
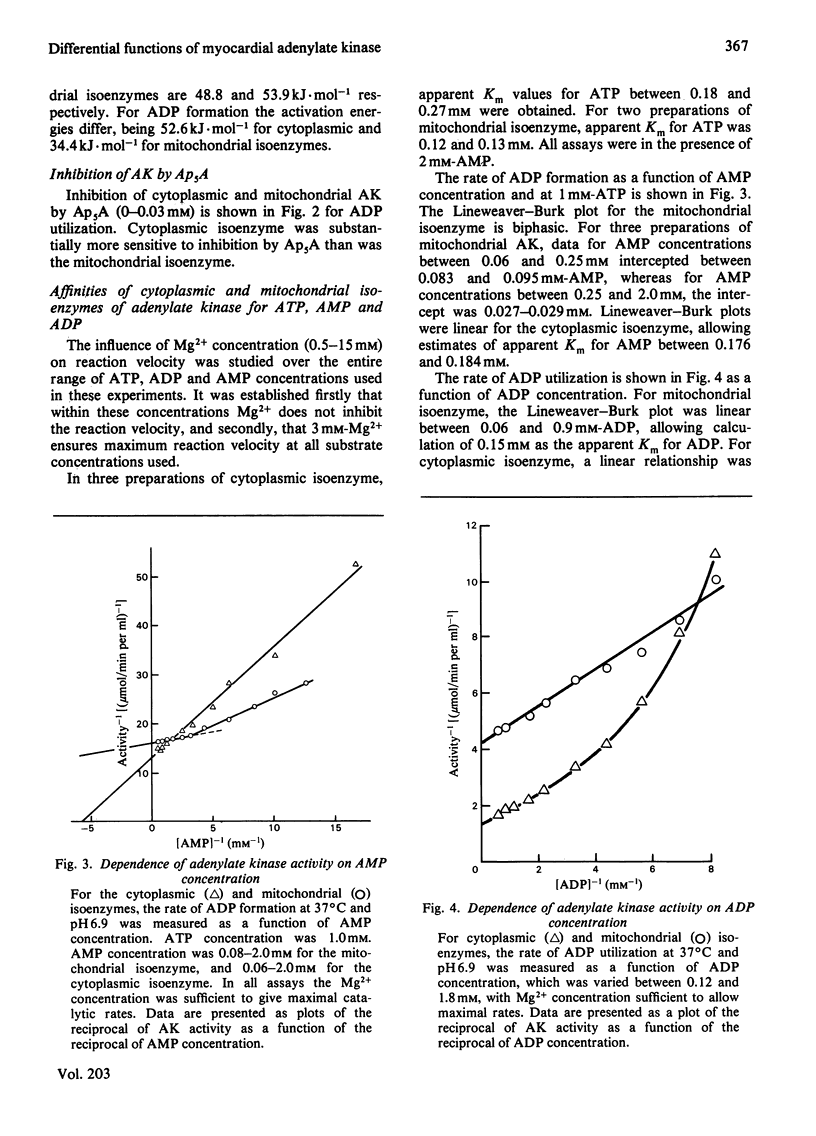
Adenylate kinase catalyses the equilibrium 2ADP = ATP + AMP. There are two isoenzymes of adenylate kinase in bovine ventricular tissue, one cytoplasmic, the other mitochondrial. Mitochondrial subfractionation locates this isoenzyme between the mitochondrial membranes with fatty acid-CoA ligase. The cytoplasmic and mitochondrial isoenzymes are distributed in ratio 3:2, and both forms were purified to homogeneity. They differ principally by charge, Km values for ATP, ADP and AMP, pH-stability and -activity profiles, and susceptibility to the inhibitor adenosine pentaphosphoadenosine. The forward and reverse reactions show similar energies of activation for the cytoplasmic enzyme, but differ for the mitochondrial enzyme. The molecular weights are indistinguishable. An integrated mechanism is formulated whereby one isoenzyme suppresses the activation of fatty acid and the other enhances carbohydrate utilization in hypoxic myocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre A., Rossi C. R., Sartorelli L., Siliprandi N. The action of atractyloside and AMP on long-chain fatty acid oxidation and on the ATP-dependent fatty acid thiokinase. FEBS Lett. 1969 Jun;3(4):279–282. doi: 10.1016/0014-5793(69)80158-4. [DOI] [PubMed] [Google Scholar]
- Bachmann E., Allmann D. W., Green D. E. The membrane systems of the mitochondrion. I. The S fraction of the outer membrane of beef heart mitochondria. Arch Biochem Biophys. 1966 Jul;115(1):153–164. doi: 10.1016/s0003-9861(66)81051-2. [DOI] [PubMed] [Google Scholar]
- CHIGA M., PLAUT G. W. Nucleotide transphosphorylases from liver. I. Purification and properties of an adenosine triphosphate-adenosine monophosphate transphosphorylase from swine liver. J Biol Chem. 1960 Nov;235:3260–3265. [PubMed] [Google Scholar]
- Criss W. E. Rat liver adenosine triphosphate: adenosine monophosphate phosphotransferase activity. II. Subcellular localization of adenylate kinase isozymes. J Biol Chem. 1970 Dec 10;245(23):6352–6356. [PubMed] [Google Scholar]
- Criss W. E., Sapico V., Litwack G. Rat liver adenosine triphosphate: adenosine monophosphate phosphotransferase activity. I. Purification and physical and kinetic characterization of adenylate kinase 3. J Biol Chem. 1970 Dec 10;245(23):6346–6351. [PubMed] [Google Scholar]
- Font B., Gautheron D. C. General and kinetic properties of pig heart mitochondrial adenylate kinase. Biochim Biophys Acta. 1980 Feb 14;611(2):299–308. doi: 10.1016/0005-2744(80)90065-0. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979 Dec 15;184(3):547–554. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. O., Dow J. W. Potassium depletion: effects on intracellular pH and electrolyte distribution in skeletal and cardiac muscle. Australas Ann Med. 1968 Aug;17(3):206–213. doi: 10.1111/imj.1968.17.3.206. [DOI] [PubMed] [Google Scholar]
- Itakura T., Watanabe K., Shiokawa H., Kubo S. Purification and characterization of acidic adenylate kinase in porcine heart. Eur J Biochem. 1978 Jan 16;82(2):431–437. doi: 10.1111/j.1432-1033.1978.tb12037.x. [DOI] [PubMed] [Google Scholar]
- Klethi J. Isolation and properties of lens adenylate kinase. Exp Eye Res. 1968 Jul;7(3):449–460. doi: 10.1016/s0014-4835(68)80061-2. [DOI] [PubMed] [Google Scholar]
- Kubo S., Noda L. H. Adenylate kinase of porcine heart. Eur J Biochem. 1974 Oct 2;48(2):325–331. doi: 10.1111/j.1432-1033.1974.tb03772.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorenson M. Y., Mansour T. E. Studies on heart phosphofructokinase. Binding properties of native enzyme and of enzyme desensitized to allosteric control. J Biol Chem. 1969 Dec 10;244(23):6420–6431. [PubMed] [Google Scholar]
- MORGAN H. E., PARMEGGIANI A. REGULATION OF GLYCOGENOLYSIS IN MUSCLE. II. CONTROL OF GLYCOGEN PHOSPHORYLASE REACTION IN ISOLATED PERFUSED HEART. J Biol Chem. 1964 Aug;239:2435–2439. [PubMed] [Google Scholar]
- Markland F. S., Wadkins C. L. Adenosine triphosphate-adenosine 5'-monophosphate phosphotransferase of bovine liver mitochondria. I. Isolation and chemical properties. J Biol Chem. 1966 Sep 25;241(18):4124–4135. [PubMed] [Google Scholar]
- NODA L. Adenosine triphosphate-adenosine monophosphate transphosphorylase. III. Kinetic studies. J Biol Chem. 1958 May;232(1):237–250. [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Whitmer J. T., Morgan H. E. Effects of ischemia on function and metabolism of the isolated working rat heart. Am J Physiol. 1973 Sep;225(3):651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Heldt H. W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim Biophys Acta. 1965 Jun 15;104(1):312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
- Pradhan T. K., Criss W. E., Morris H. P. Kinetic regulation of adenylate kinases from muscle, liver, and hepatoma. Cancer Res. 1974 Nov;34(11):3058–3061. [PubMed] [Google Scholar]
- REGEN D. M., DAVIS W. W., MORGAN H. E., PARK C. R. THE REGULATION OF HEXOKINASE AND PHOSPHOFRUCTOKINASE ACTIVITY IN HEART MUSCLE. EFFECTS OF ALLOXAN DIABETES, GROWTH HORMONE, CORTISOL, AND ANOXIA. J Biol Chem. 1964 Jan;239:43–49. [PubMed] [Google Scholar]
- Sapico V., Litwack G., Criss W. E. Purification of rat liver adenylate kinase isozyme II and comparison with isozyme 3. Biochim Biophys Acta. 1972 Feb 28;258(2):436–445. doi: 10.1016/0005-2744(72)90235-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Purification of glycolytic enzymes by using affinity-elution chromatography. Biochem J. 1977 Feb 1;161(2):253–263. doi: 10.1042/bj1610253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Suzue G., Marcel Y. L. Specificity of long-chain acyl coenzyme A synthetase from rat liver microsomes. Influence of the position of double bonds in octadecadienoic acids. Biochemistry. 1972 Apr 25;11(9):1704–1708. doi: 10.1021/bi00759a027. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Tomasselli A. G., Noda L. H. Mitochondrial ATP:AMP phosphotransferase from beef heart: purification and properties. Eur J Biochem. 1980 Feb;103(3):481–491. doi: 10.1111/j.1432-1033.1980.tb05972.x. [DOI] [PubMed] [Google Scholar]


