Abstract
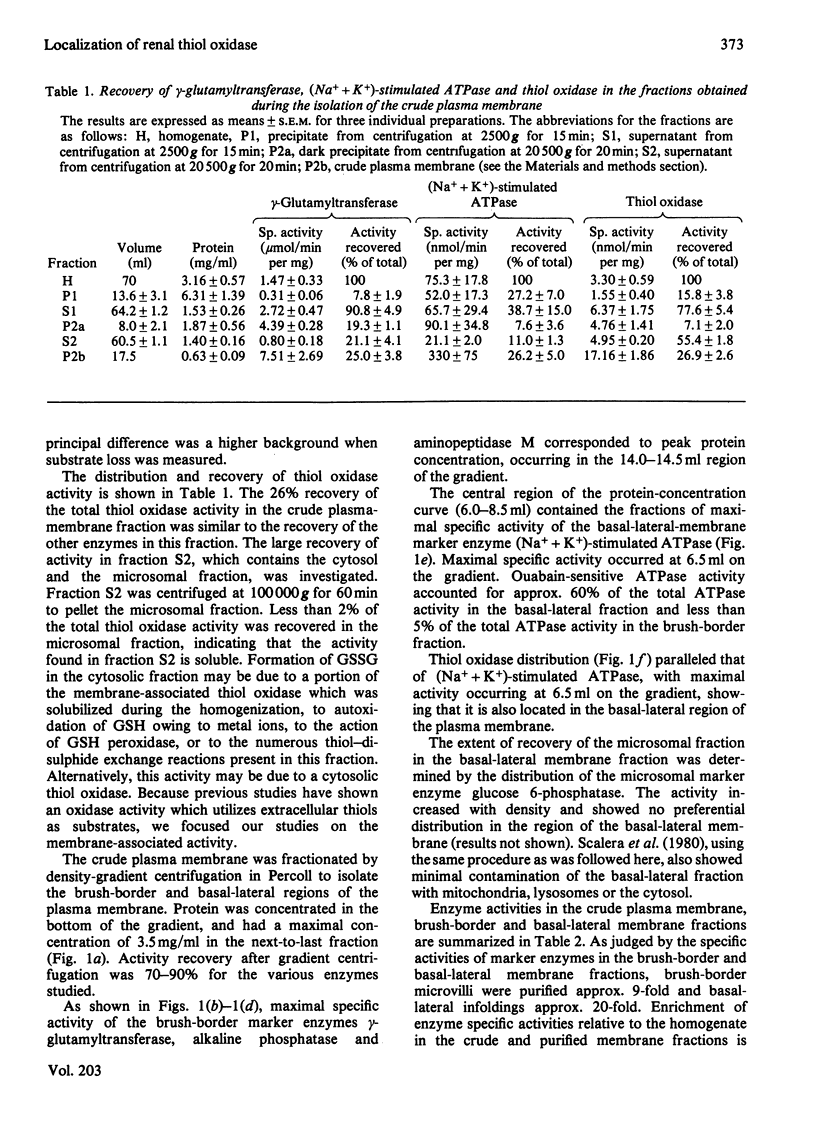
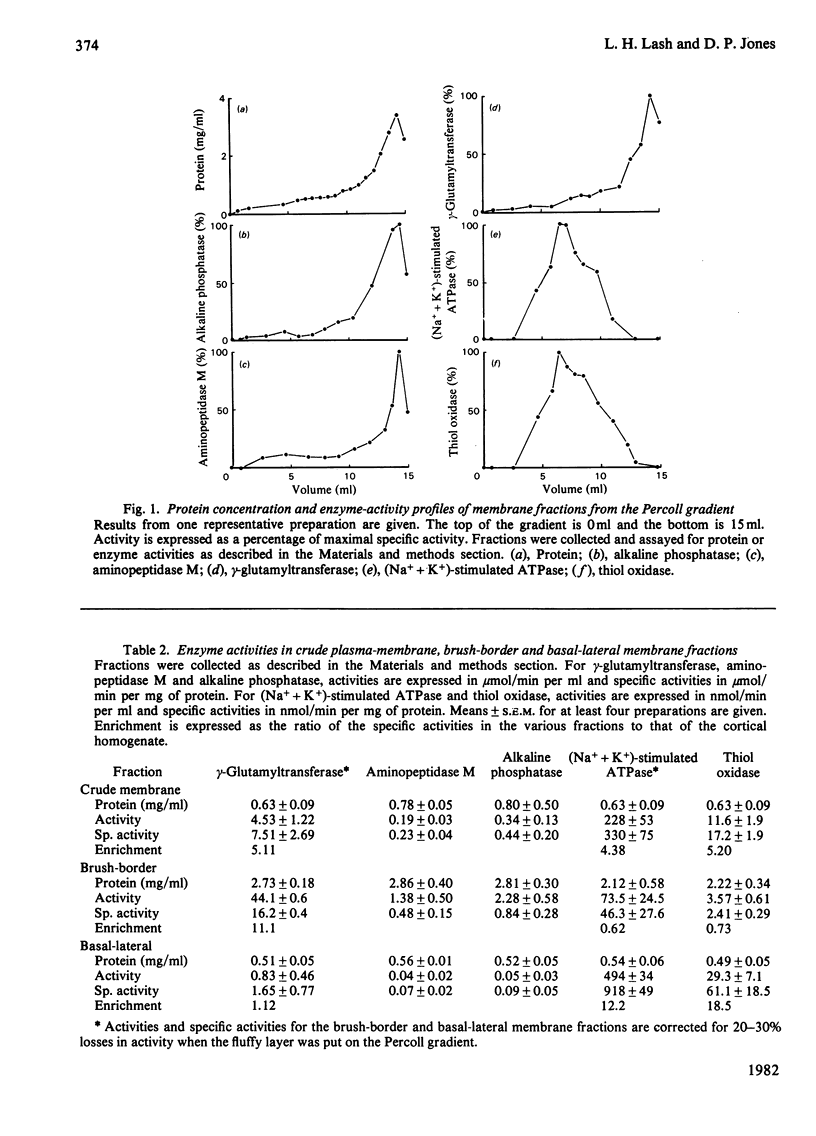
The localization of the membrane-associated thiol oxidase in rat kidney was investigated. Fractionation of the kidney cortex by differential centrifugation demonstrated that the enzyme is found in the plasma membrane. The crude plasma membrane was fractionated by density-gradient centrifugation on Percoll to obtain purified brush-border and basal-lateral membranes. Gamma-Glutamyltransferase, alkaline phosphatase and aminopeptidase M were assayed as brush-border marker enzymes, and (Na+ + K+)-stimulated ATPase was assayed as a basal-lateral-membrane marker enzyme. Thiol oxidase activity and distribution were determined and compared with those of the marker enzymes. Its specific activity was enriched 18-fold in the basal-lateral membrane fraction relative to its activity in the cortical homogenate, and its distribution paralleled that of (Na+ + K+)-stimulated ATPase. This association indicates that thiol oxidase is localized in the same fraction as (Na+ + K+)-stimulated ATPase, i.e. the basal-lateral region of the plasma membrane of the kidney tubular epithelium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkar S., Binkley F., Jones D. P. Resolution of a renal sulfhydryl (glutathione) oxidase from gamma-glutamyltransferase. FEBS Lett. 1981 Feb 23;124(2):166–172. doi: 10.1016/0014-5793(81)80128-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- GLENNER G. G., FOLK J. E. Glutamyl peptidases in rat and guinea pig kidney slices. Nature. 1961 Oct 28;192:338–340. doi: 10.1038/192338a0. [DOI] [PubMed] [Google Scholar]
- GOLDFISCHER S., ESSNER E., NOVIKOFF A. B. THE LOCALIZATION OF PHOSPHATASE ACTIVITIES AT THE LEVEL OF ULTRASTRUCTURE. J Histochem Cytochem. 1964 Feb;12:72–95. doi: 10.1177/12.2.72. [DOI] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M. gamma-Glutamyltransferase in kidney brush border membranes. FEBS Lett. 1972 Jan 1;19(4):340–344. doi: 10.1016/0014-5793(72)80075-9. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Tate S. S. The apparent glutathione oxidase activity of gamma-glutamyl transpeptidase. Chemical mechanism. J Biol Chem. 1980 Jun 10;255(11):5011–5014. [PubMed] [Google Scholar]
- HOLT J. H., MILLER D. The localization of phosphomonoesterase and aminopeptidase in brush borders isolated from intestinal epithelial cells. Biochim Biophys Acta. 1962 Apr 9;58:239–243. doi: 10.1016/0006-3002(62)91004-1. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Janolino V. G., Swaisgood H. E. Isolation and characterization of sulfhydryl oxidase from bovine milk. J Biol Chem. 1975 Apr 10;250(7):2532–2538. [PubMed] [Google Scholar]
- Jones D. P., Moldfus P., Stead A. H., Ormstad K., Jörnvall H., Orrenius S. Metabolism of glutathione and a glutathione conjugate by isolated kidney cells. J Biol Chem. 1979 Apr 25;254(8):2787–2792. [PubMed] [Google Scholar]
- Liang C. T., Sacktor B. Preparation of renal cortex basal-lateral and bursh border membranes. Localization of adenylate cyclase and guanylate cyclase activities. Biochim Biophys Acta. 1977 May 2;466(3):474–487. doi: 10.1016/0005-2736(77)90340-6. [DOI] [PubMed] [Google Scholar]
- MOELBERT E. R., DUSPIVA F., von DEIMLING O. The demonstration of alkaline phosphatase in the electron microscope. J Biophys Biochem Cytol. 1960 Apr;7:387–390. [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., MONIS B., ROSENBATT D., SELIGMAN A. M. Improvement in the histochemical localization of leucine aminopeptidase with a new substrate, L-leucyl-4-methoxy-2-naphthylamide. J Biophys Biochem Cytol. 1960 Apr;7:261–264. doi: 10.1083/jcb.7.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- Ormstad K., Moldéus P., Orrenius S. Partial characterization of a glutathione oxidase present in rat kidney plasma membrane fraction. Biochem Biophys Res Commun. 1979 Jul 27;89(2):497–503. doi: 10.1016/0006-291x(79)90657-0. [DOI] [PubMed] [Google Scholar]
- Ormstad K., Orrenius S. The localization of renal glutathione oxidase activity studied in the isolated, perfused rat kidney. Biochem Biophys Res Commun. 1980 Jan 29;92(2):540–545. doi: 10.1016/0006-291x(80)90367-8. [DOI] [PubMed] [Google Scholar]
- Ostrowski M. C., Kistler W. S. Properties of a flavoprotein sulfhydryl oxidase from rat seminal vesicle secretion. Biochemistry. 1980 Jun 10;19(12):2639–2645. doi: 10.1021/bi00553a016. [DOI] [PubMed] [Google Scholar]
- REVEL J. P., BALL E. G. The reaction of glutathione with amino acids and related compounds as catalyzed by gamma-glutamyl transpeptidase. J Biol Chem. 1959 Mar;234(3):577–582. [PubMed] [Google Scholar]
- Reed D. J., Ellis W. W., Meck R. A. The inhibition of gamma-glutamyl transpeptidase and glutathione metabolism of isolated rat kidney cells by L-(alpha S, 5S)-alpha-amino-3-chloro-4, 5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Biochem Biophys Res Commun. 1980 Jun 30;94(4):1273–1277. doi: 10.1016/0006-291x(80)90557-4. [DOI] [PubMed] [Google Scholar]
- Scalera V., Storelli C., Storelli-Joss C., Haase W., Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J. 1980 Jan 15;186(1):177–181. doi: 10.1042/bj1860177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Dubach U. C. Na K stimulated adenosinetriphosphatase: intracellular localisation within the proximal tubule of the rat nephron. Pflugers Arch. 1971;330(3):265–270. doi: 10.1007/BF00588617. [DOI] [PubMed] [Google Scholar]
- Schoner W., von Ilberg C., Kramer R., Seubert W. On the mechanism of Na+- and K+-stimulated hydrolysis of adenosine triphosphate. 1. Purification and properties of a Na+-and K+-activated ATPase from ox brain. Eur J Biochem. 1967 May;1(3):334–343. doi: 10.1007/978-3-662-25813-2_45. [DOI] [PubMed] [Google Scholar]
- Szasz G. A kinetic photometric method for serum leucine aminopeptidase. Am J Clin Pathol. 1967 May;47(5):607–613. doi: 10.1093/ajcp/47.5.607. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Orlando J. Conversion of glutathione to glutathione disulfide, a catalytic function of gamma-glutamyl transpeptidase. J Biol Chem. 1979 Jul 10;254(13):5573–5575. [PubMed] [Google Scholar]
- Wilfong R. F., Neville D. M., Jr The isolation of a brush border membrane fraction from rat kidney. J Biol Chem. 1970 Nov 25;245(22):6106–6112. [PubMed] [Google Scholar]


