Abstract
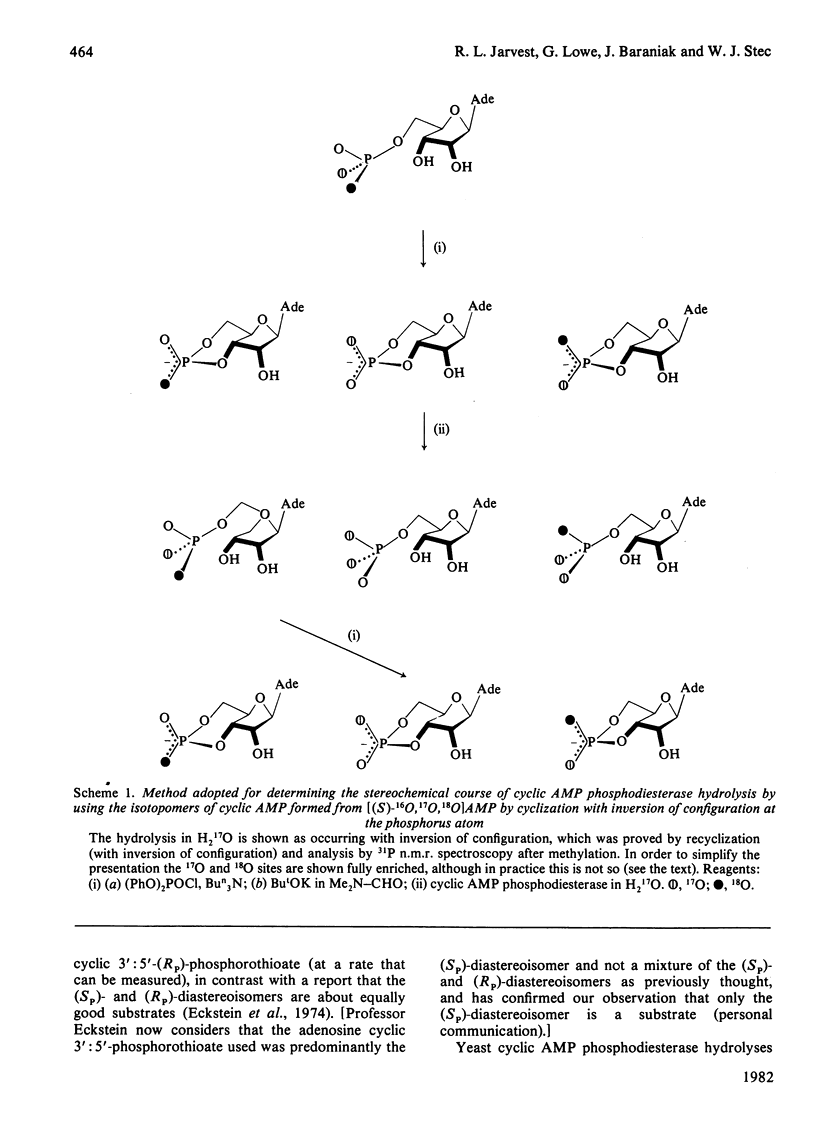
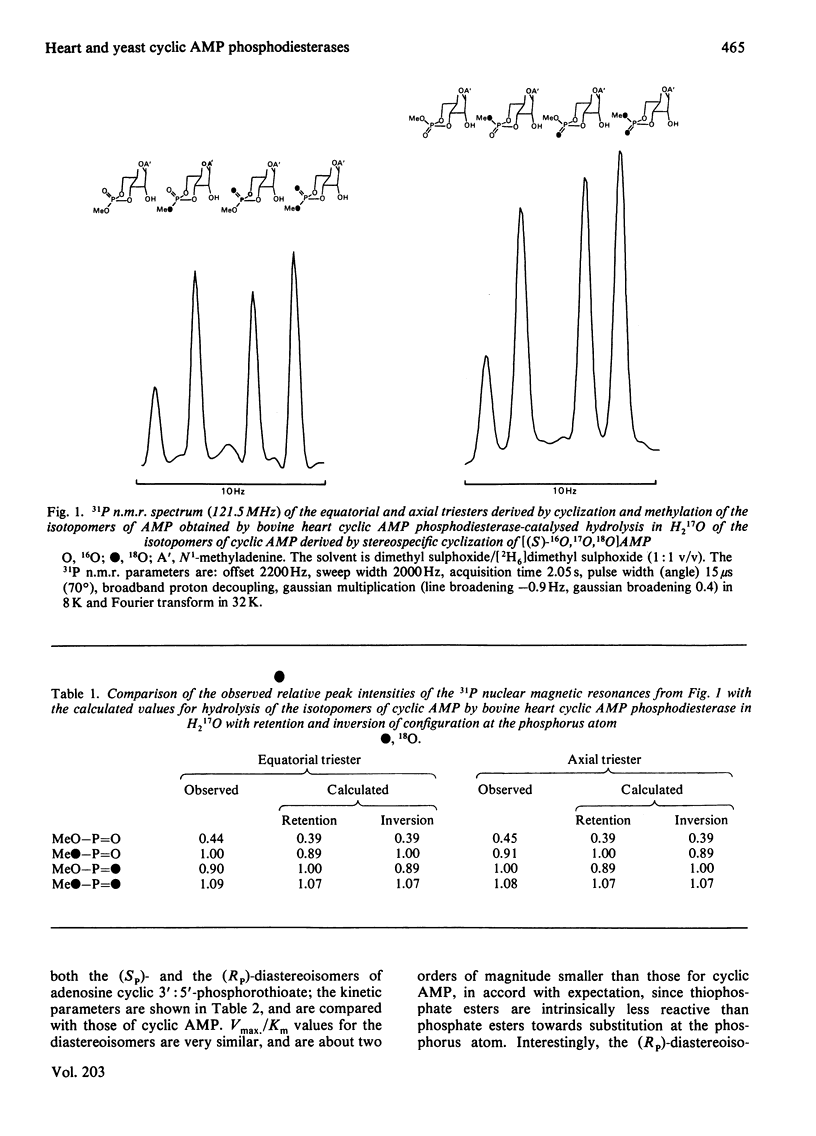
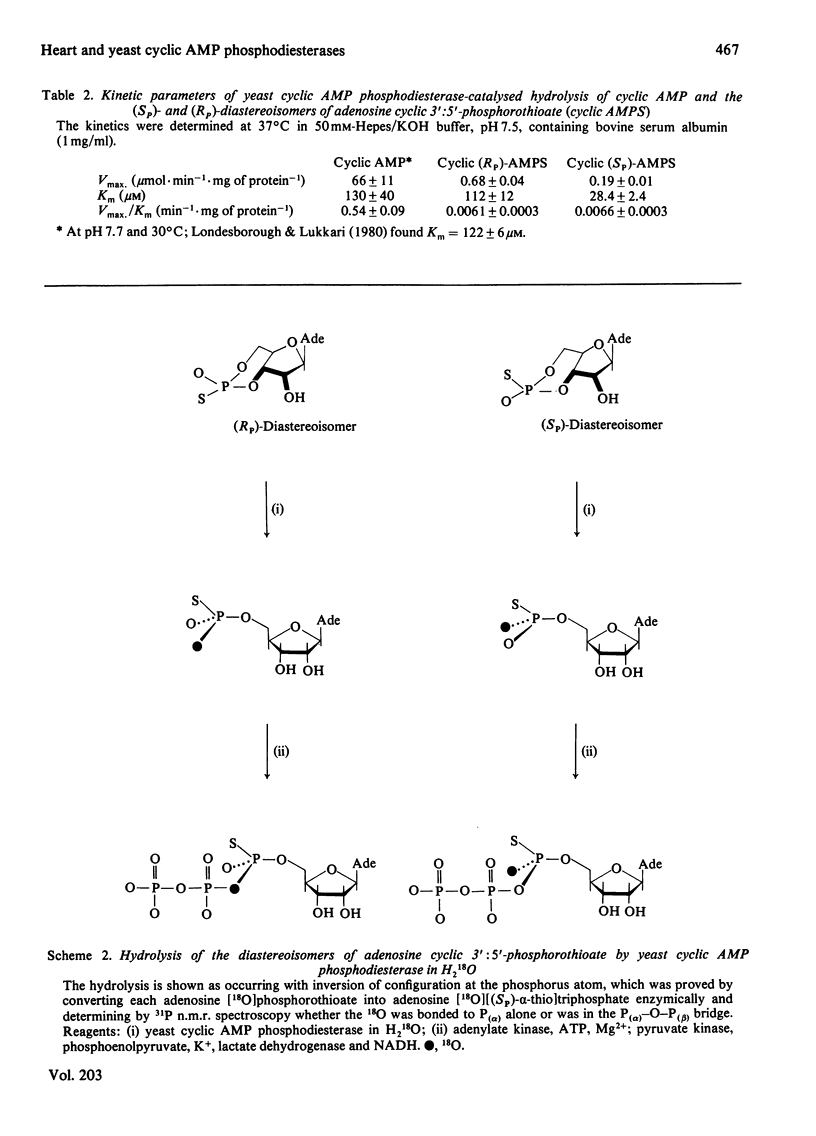
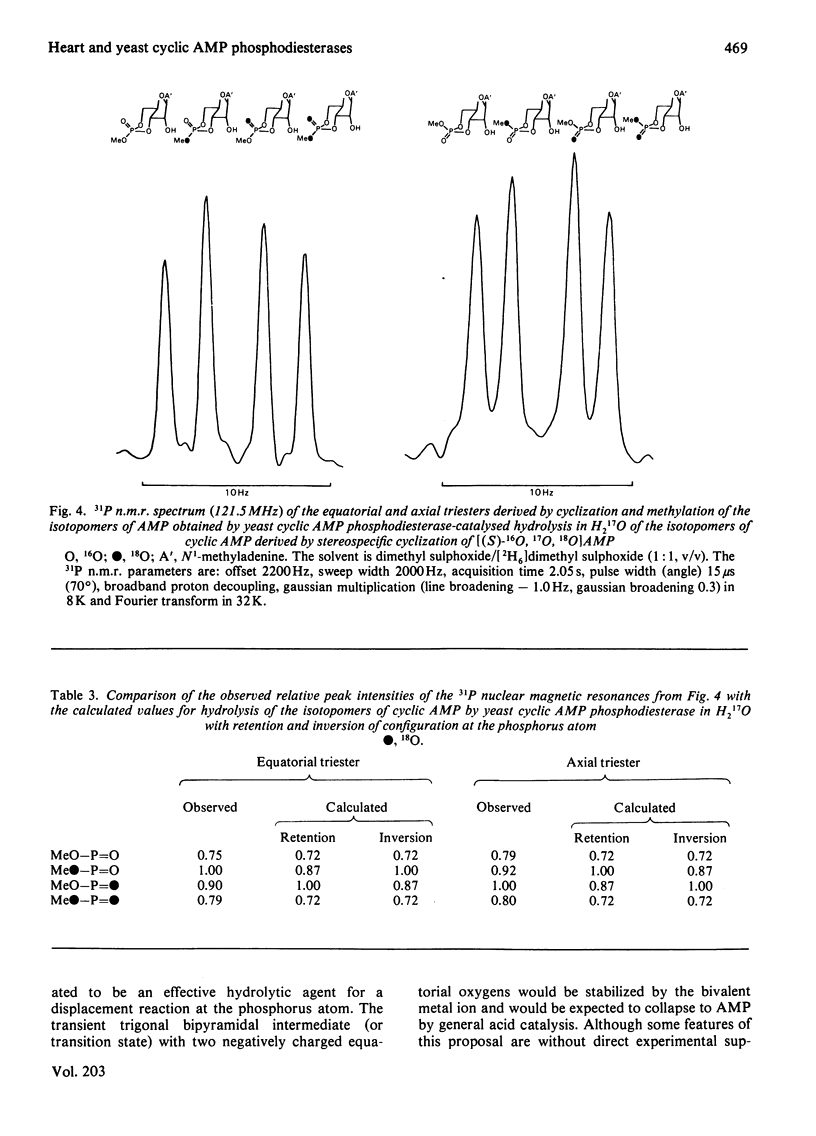
Bovine heart cyclic AMP phosphodiesterase, which has a requirement for Mg2+, hydrolyses cyclic AMP with inversion of configuration at the phosphorus atom, but only the (Sp)-diastereoisomer of adenosine cyclic 3':5'-phosphorothioate is hydrolysed by this enzyme. By contrast, the low-affinity yeast cyclic AMP phosphodiesterase, which contains tightly bound Zn2+, hydrolyses both the (Sp)- and the (Rp)-diastereoisomers of adenosine cyclic 3':5'-phosphorothioate, the (Rp)-diastereoisomer being the preferred substrate under V max. conditions. Both of the diastereoisomers of adenosine cyclic 3':5'-phosphorothioate, as well as cyclic AMP, are hydrolysed with inversion of configuration at the phosphorus atom by the yeast enzyme. It is proposed that, with both enzymes, the bivalent metal ion co-ordinates with the phosphate residue of the substrate, and that hydrolysis is catalysed by a direct "in-line' mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F., Hunneman D. H., Baraniak J., Kinas R. W., Lesiak K., Stec W. J. Stereochemistry of hydrolysis of adenosine 3':5'-cyclic phosphorothioate by the cyclic phosphodiesterase from beef heart. J Biol Chem. 1979 Oct 25;254(20):9959–9961. [PubMed] [Google Scholar]
- Cornelius R. D., Cleland W. W. Substrate activity of (adenosine triphosphato)tetraamminecobalt(III) with yeast hexokinase and separation of diastereomers using the enzyme. Biochemistry. 1978 Aug 8;17(16):3279–3286. doi: 10.1021/bi00609a016. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Simonson L. P., Bär H. P. Adenosine 3',5'-cyclic phosphorothioate: synthesis and biological properties. Biochemistry. 1974 Aug 27;13(18):3806–3810. doi: 10.1021/bi00715a029. [DOI] [PubMed] [Google Scholar]
- Gerlt J. A., Coderre J. A., Wolin M. S. Mechanism of the adenylate cyclase reaction. Stereochemistry of the reaction catalyzed by the enzyme from Brevibacterium liquefaciens. J Biol Chem. 1980 Jan 25;255(2):331–334. [PubMed] [Google Scholar]
- Jaffe E. K., Cohn M. Diastereomers of the nucleoside phosphorothioates as probes of the structure of the metal nucleotide substrates and of the nucleotide binding site of yeast hexokinase. J Biol Chem. 1979 Nov 10;254(21):10839–10845. [PubMed] [Google Scholar]
- Londesborough J., Lukkari T. M. The pH and temperature dependence of the activity of the high Km cyclic nucleotide phosphodiesterase of bakers' yeast. J Biol Chem. 1980 Oct 10;255(19):9262–9267. [PubMed] [Google Scholar]
- Londesborough J. The high-Km cyclic AMP phosphodiesterase of baker's yeast is a zinc metalloenzyme [proceedings]. Biochem Soc Trans. 1978;6(6):1218–1220. doi: 10.1042/bst0061218. [DOI] [PubMed] [Google Scholar]
- Rex Sheu K. F., Frey P. A. Enzymatic and 32P nuclear magnetic resonance study of adenylate kinase-catalyzed stereospecific phosphorylation of adenosine 5'-phosphorothioate. J Biol Chem. 1977 Jul 10;252(13):4445–4448. [PubMed] [Google Scholar]
- Sutherland E. W. Studies on the mechanism of hormone action. Science. 1972 Aug 4;177(4047):401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- Tsai M. D. Use of phosphorus-31 nuclear magnetic resonance to distinguish bridge and nonbridge oxygens of oxygen-17-enriched nucleoside triphosphates. Stereochemistry of acetate activation by acetyl coenzyme A synthetase. Biochemistry. 1979 Apr 17;18(8):1468–1472. doi: 10.1021/bi00575a013. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Hardman J. G. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1977;8:119–143. [PubMed] [Google Scholar]


