Abstract
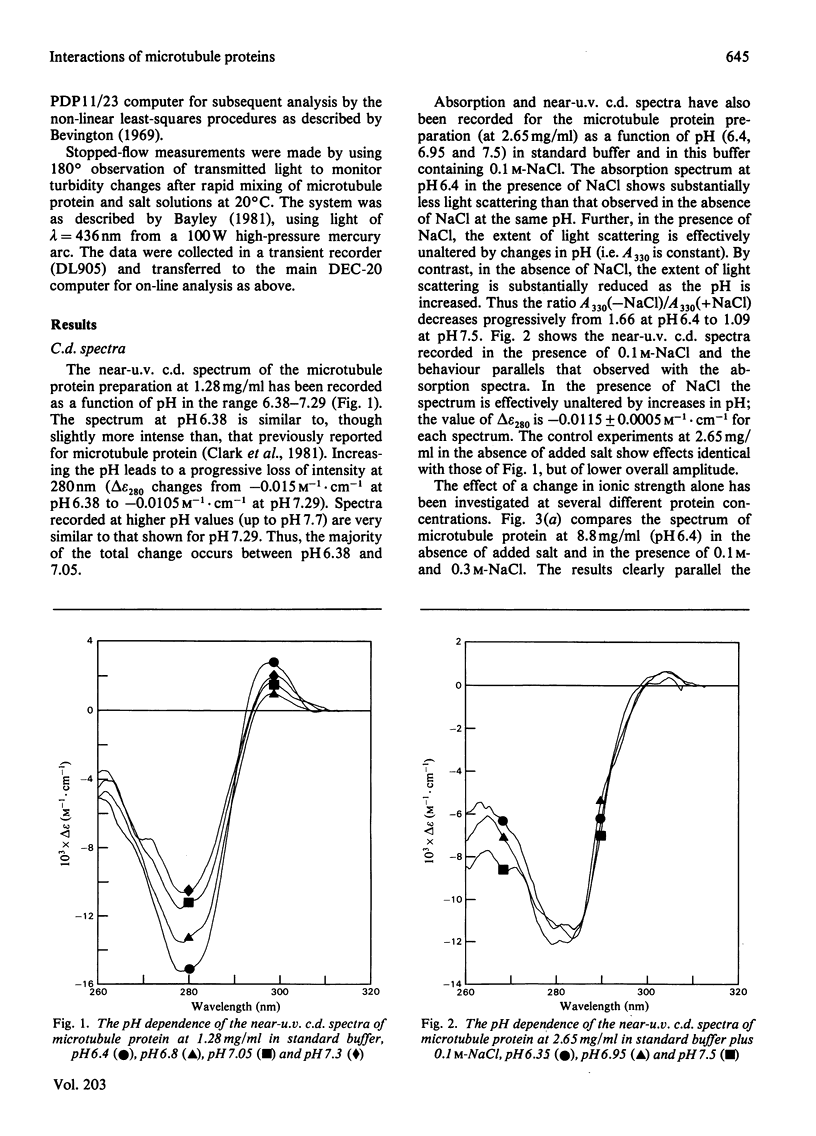
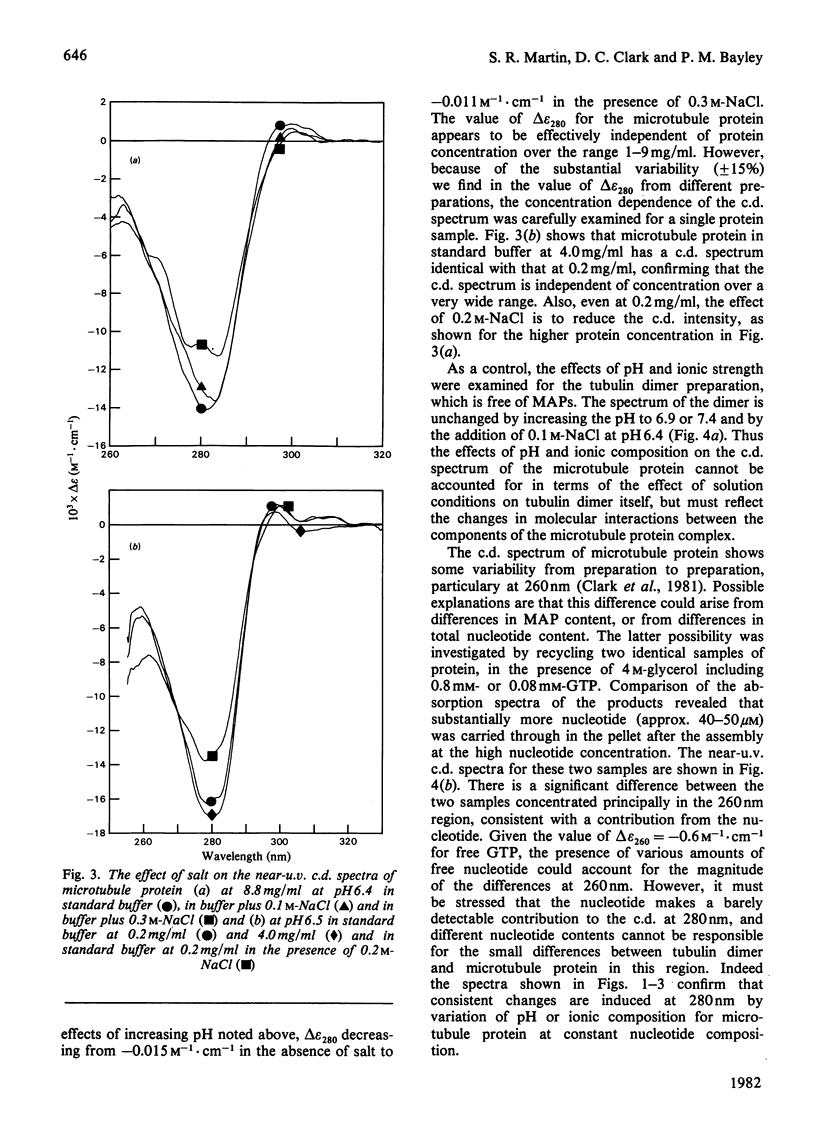
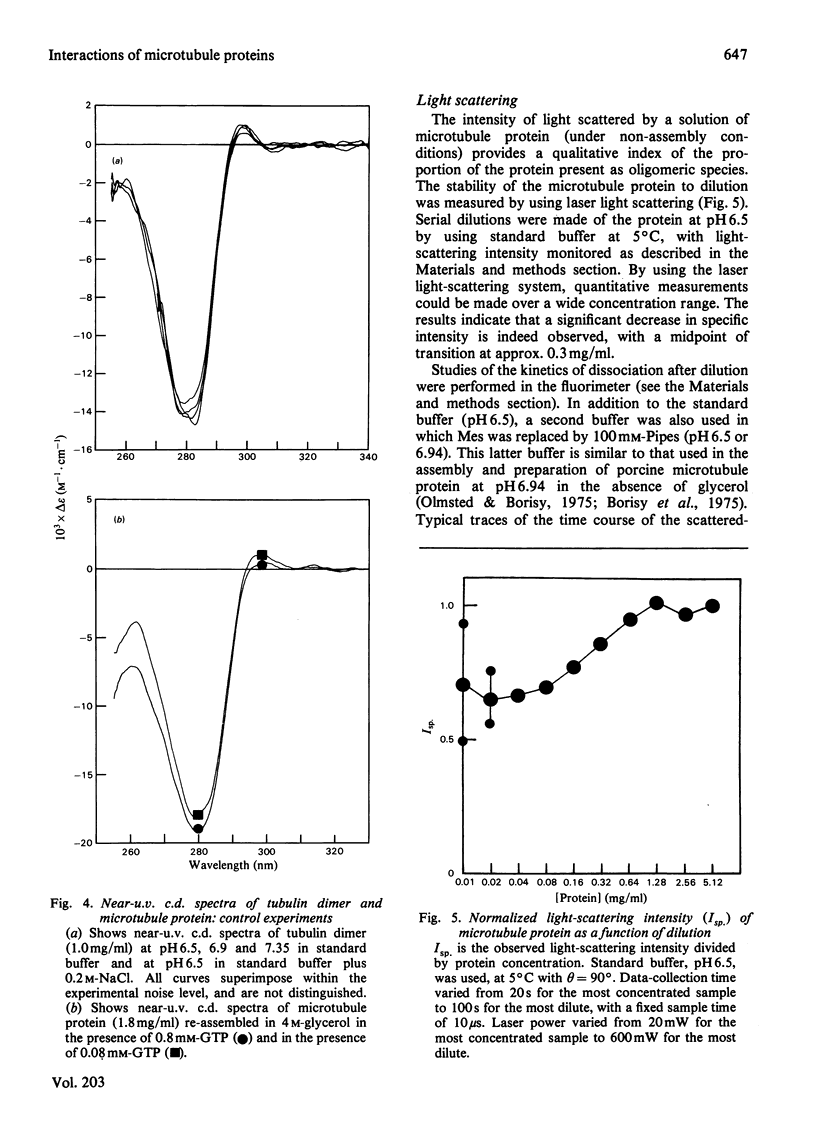
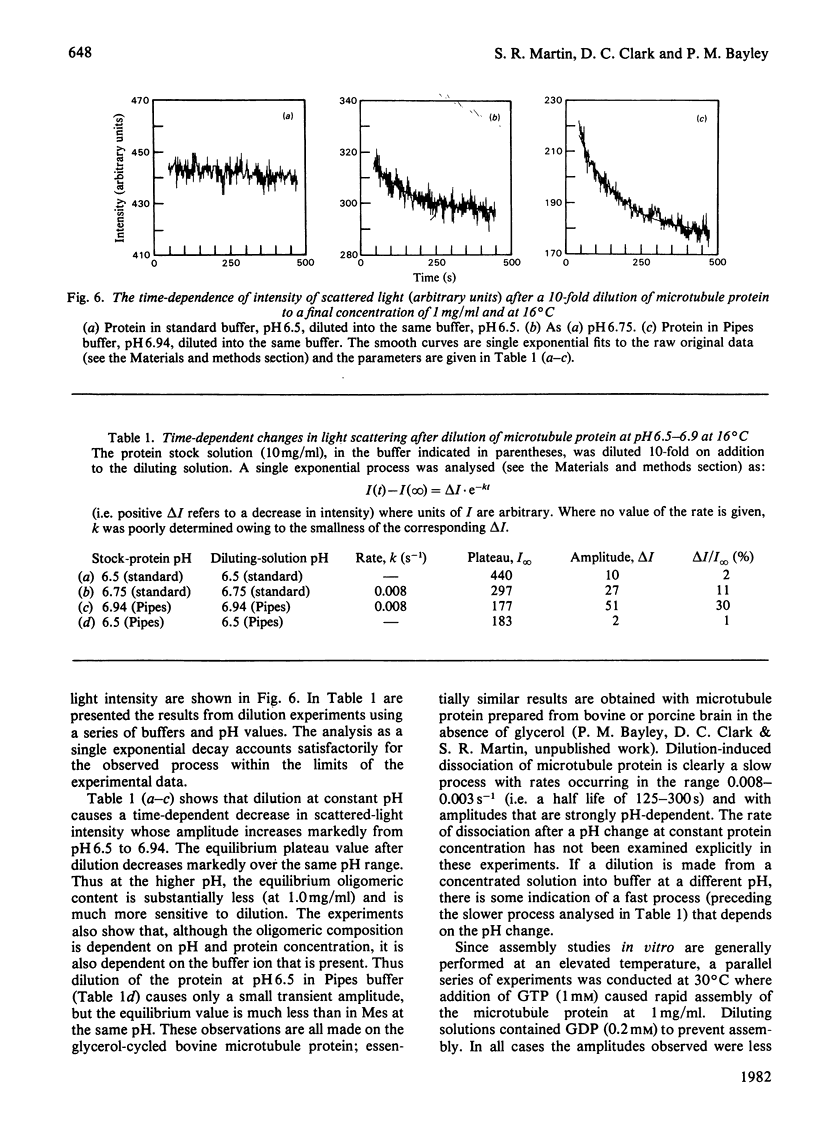
1. The conformation of bovine microtubule protein prepared by cycles of assembly and disassembly in the presence of glycerol has been studied by near-u.v. circular dichroism (c.d.) over a range of protein concentrations. The effects on the conformational properties of ionic strength and of a pH range from 6 to 7.5 have been correlated with the known oligomeric composition of microtubule protein preparations, as determined by the sedimentation behaviour of this preparation [Bayley, Charlwood, Clark & Martin (1982) Eur. J. Biochem. 121, 579–585]. 2. The formation of 30S oligomeric ring species, either by decreasing ionic strength at pH6.5 or by changing pH in the presence of 0.1m-NaCl, correlates with a significant change in tubulin c.d. Formation of 18S oligomer by changing pH at ionic strength 0.2 produced no comparable effect. The c.d. of tubulin dimer itself is not affected by ionic strength and pH over the same range. 3. The results are interpreted as a small conformational adjustment between tubulin and specific microtubule-associated proteins on forming 30S oligomeric species, due to interaction with the high-molecular-weight-group proteins. The possible significance of this is discussed with respect to microtubule assembly in vitro. 4. By using this conformational parameter, together with equilibrium and kinetic light-scattering studies, the sensitivity of glycerol-cycled microtubule protein to dilution is shown to be strongly pH-dependent, the oligomers being much more stable at pH6.4 than at pH6.9. 5. Oligomeric complexes of tubulin with microtubule-associated proteins show marked stability under conditions similar to those for efficient microtubule assembly in vitro. Oligomeric material therefore must be incorporated directly during assembly in vitro from microtubule protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asnes C. F., Wilson L. Isolation of bovine brain microtubule protein without glycerol: polymerization kinetics change during purification cycles. Anal Biochem. 1979 Sep 15;98(1):64–73. doi: 10.1016/0003-2697(79)90706-1. [DOI] [PubMed] [Google Scholar]
- Bayley P. M., Charlwood P. A., Clark D. C., Martin S. R. Oligomeric species in glycerol-cycled bovine-brain microtubule protein. Analytical ultracentrifugal characterisation. Eur J Biochem. 1982 Jan;121(3):579–585. doi: 10.1111/j.1432-1033.1982.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Bryan J. A quantitative analysis of microtubule elongation. J Cell Biol. 1976 Dec;71(3):749–767. doi: 10.1083/jcb.71.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. C., Martin S. R., Bayley P. M. A study of tubulin dimer conformation by near-UV circular dichroism. Biochem Biophys Res Commun. 1980 Nov 28;97(2):628–634. doi: 10.1016/0006-291x(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Clark D. C., Martin S. R., Bayley P. M. Conformation and assembly characteristics of tubulin and microtubule protein from bovine brain. Biochemistry. 1981 Mar 31;20(7):1924–1932. doi: 10.1021/bi00510a031. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Assembly of microtubules from preformed, ring-shaped protofilaments and 6-S tubulin. J Supramol Struct. 1974;2(2-4):393–411. doi: 10.1002/jss.400020228. [DOI] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry. 1975 Oct 21;14(21):4559–4566. doi: 10.1021/bi00692a001. [DOI] [PubMed] [Google Scholar]
- Goux W. J., Hooker T. M., Jr The chiroptical properties of proteins. II. Near-ultraviolet circular dichroism of lysozyme. Biopolymers. 1980 Dec;19(12):2191–2208. doi: 10.1002/bip.1980.360191205. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977 Nov 25;117(1):1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- Karr T. L., Purich D. L. Rings are not microtubule assembly intermediates: an analysis of the lag phase in GTP-dependent self-assembly of bovine brain tubulin. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1885–1889. doi: 10.1016/s0006-291x(80)80119-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Corfman D., Frigon R. P., Timasheff S. N. Conformational study of calf brain tubulin. Arch Biochem Biophys. 1978 Jan 15;185(1):4–14. doi: 10.1016/0003-9861(78)90137-6. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Field D. J., Lee L. L. Effects of nocodazole on structures of calf brain tubulin. Biochemistry. 1980 Dec 23;19(26):6209–6215. doi: 10.1021/bi00567a041. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry. 1977 Apr 19;16(8):1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Borisy G. G. Characterization of microtubule protein oligomers by analytical ultracentrifugation. J Biol Chem. 1978 Apr 25;253(8):2825–2833. [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Sattelle D. B., Palmer G. R., Griffin M. C., Holder R. E. Spectrometer for laser-light scattering measurements of macromolecular and subcellular particle motions. Med Biol Eng Comput. 1982 Jan;20(1):37–43. doi: 10.1007/BF02441848. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Borisy G. G. Comparison of the sedimentation properties of microtubule protein oligomers prepared by two different procedures. Biochem Biophys Res Commun. 1976 May 3;70(1):1–7. doi: 10.1016/0006-291x(76)91100-1. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978 Apr 25;253(8):2834–2845. [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Timasheff S. N. Aggregation of microtubule subunit protein. Effects of divalent cations, colchicine and vinblastine. Biochemistry. 1970 Oct 13;9(21):4110–4116. doi: 10.1021/bi00823a012. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Detrich H. W., 3rd Separation of tubulin from microtubule-associated proteins on phosphocellulose. Accompanying alterations in concentrations of buffer components. Biochemistry. 1979 Jun 12;18(12):2499–2503. doi: 10.1021/bi00579a010. [DOI] [PubMed] [Google Scholar]


