Abstract
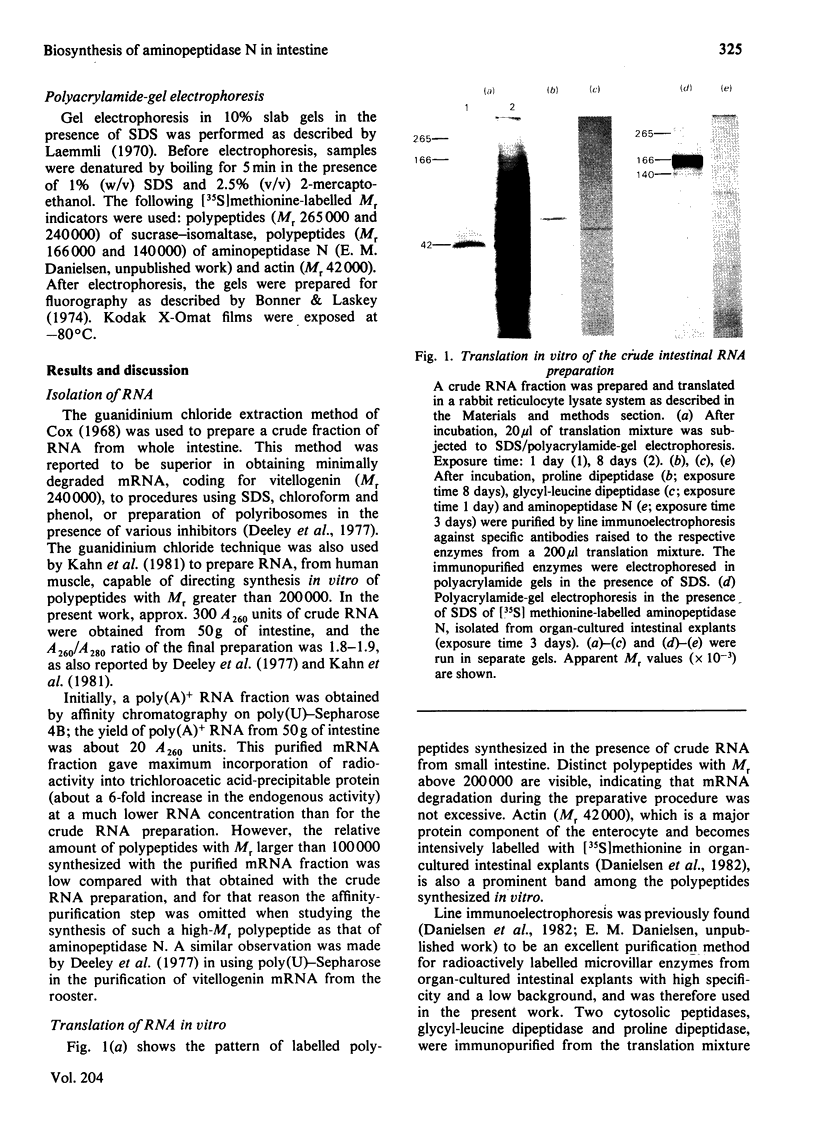
A crude RNA fraction, prepared from pig small intestine, was found to be more efficient than a fraction enriched in polyadenylated RNA in directing translation of polypeptides with Mr greater than 100000 in a rabbit reticulocyte lysate system. Aminopeptidase N (EC 3.4.11.2) synthesized in vitro was immunopurified from the translation mixture and analysed by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. It was found to have an apparent Mr of 115000 regardless of whether the translation was performed in the absence or presence of proteinase inhibitors. This result contradicts the possibility of aminopeptidase N being synthesized as a large single-chain precursor polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Bro B., Dabelsteen E. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J. 1982 Mar 15;202(3):647–654. doi: 10.1042/bj2020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Skovbjerg H., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Nature of precursor forms of microvillar enzymes from Ca2+-precipitated enterocyte membranes. FEBS Lett. 1981 Sep 28;132(2):197–200. doi: 10.1016/0014-5793(81)81159-3. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Norén O., Dabelsteen E., Sjöström H., Josefsson L. Histological localization of two dipeptidases in the pig small intestine and liver, using immunofluorescence. Gastroenterology. 1977 Jan;72(1):87–92. [PubMed] [Google Scholar]
- Norén O., Sjöström H., Josefsson L. Studies on a soluble dipeptidase from pig intestinal mucosa. I. Purification and specificity. Biochim Biophys Acta. 1973 Dec 19;327(2):446–456. doi: 10.1016/0005-2744(73)90428-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Christiansen L., Wacker H., Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem. 1980 Dec 10;255(23):11332–11338. [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Josefsson L. Purification and specificity of pig intestinal prolidase. Biochim Biophys Acta. 1973 Dec 19;327(2):457–470. doi: 10.1016/0005-2744(73)90429-4. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O. Size and shape of two intestinal dipeptidases. Int J Pept Protein Res. 1978 Feb;11(2):159–165. doi: 10.1111/j.1399-3011.1978.tb02835.x. [DOI] [PubMed] [Google Scholar]
- Wacker H., Jaussi R., Sonderegger P., Dokow M., Ghersa P., Hauri H. P., Christen P., Semenza G. Cell-free synthesis of the one-chain precursor of a major intrinsic protein complex of the small-intestinal brush border membrane (pro-sucrase-isomaltase). FEBS Lett. 1981 Dec 28;136(2):329–332. doi: 10.1016/0014-5793(81)80647-3. [DOI] [PubMed] [Google Scholar]