Abstract
Resin cement exhibits numerous therapeutic advantages over conventional luting materials. However, the effectiveness of the antibacterial properties of resin cement remains unclear. Nanotechnology provides a viable option, whereby the integration of nanoparticles (NPs) can potentially augment the antibacterial effectiveness of resin cement. The objective of this study was to conduct a comprehensive literature review to assess resin cement’s antibacterial effectiveness by incorporating nanoparticles. An extensive search of PubMed and Scopus databases up to September 12, 2023, was conducted to identify relevant scholarly articles that examined and evaluated resin cement’s antibacterial effectiveness with and without the incorporation of nanoparticles (NPs). This systematic review adhered to the PRISMA guidelines for reporting the results. The search retrieved seven eligible studies. Studies indicated that resin cement with NPs significantly reduced the colony forming unit (CFU) counts compared to resin cement without NPs. Furthermore, resin cement, in addition to NPs, significantly reduced the bacterial metabolic activity compared to the control group. The use of nanoparticles (NPs) in resin cement has been shown to enhance its antibacterial properties, possibly mitigating the occurrence of secondary caries. Future clinical trials are required to validate the beneficial effects of NPs in conjunction with resin cement in the prevention of secondary caries.
Keywords: Material science, Resin cement, Nanoparticles, Antibacterial, Nanomaterials
Introduction
Resin cement is an innovative luting material that has been developed specifically for dental applications [1]. The composition includes a resin matrix comprising of constituents such as urethane dimethacrylate and bisphenol A-glycidyl methacrylate (Bis-GMA) in addition to finely dispersed inorganic filler particles. Resin cement is distinguished by its characteristics such as low viscosity and optimized distribution of fillers, rendering it a composite material capable of achieving a thin film [2, 3]. Resin cement can be classified into three categories based on its polymerization mechanism: light-cured, chemical-cured, and dual-cured. Furthermore, they can be categorized according to their adhesive scheme, which includes total-etch, self-etching, and self-adhesive approaches [4–7]. They exhibit remarkable versatility in a wide range of dental applications encompassing the cementation of various dental restorations, such as full-cast metal crowns, ceramic crowns, zirconia constructions, indirect composite restorations, traditional metal-ceramic constructions, metal and glass fiber posts, implant-supported crowns and bridges, and ceramic veneers [8–13].
Resin cements are known to have superior mechanical and physical properties compared to the other luting materials used in past [14, 15]. They exhibit excellent compression resistance, improved hardness, and good flexural strength. In addition, resin cements have substantial strength against fatigue and demonstrate the ability to bond well with a diverse array of materials. Furthermore, these dental restorations can modify the shade and color, have excellent durability, display resistance to wear at the edge of the restorations, and exhibit minimal marginal permeability [16–19]. Nevertheless, it is crucial to consider that resin cement may exhibit a comparatively lower capacity to limit caries progression when juxtaposed with alternative luting materials, such as glass-ionomer cements. This raises concerns regarding their potential role in the inhibition of secondary caries [20, 21].
Nanotechnology has emerged as a promising field of study in dental research, exhibiting a significant potential for augmenting the antibacterial and mechanical characteristics of dental materials [22–25]. By incorporating nanoparticles (NPs) into dental materials such as silver nanoparticles (Ag Nps), it is possible to create materials that effectively inhibit bacterial buildup and the development of secondary caries [26–30]. It has been observed that adding Ag Nps to resin cements can be beneficial in retarding bacterial growth [31]. The properties of nanoparticles such as large surface area and high density, assist in enhancing the antimicrobial properties, as they can easily interact with negatively charged bacterial cells [32]. It has been observed that the Ag Nps can be effective in retarding the growth of Streptococcus mutans, the most common cariogenic bacteria [31]. The growth of other bacteria such as Staphylococcus aureus, Streptococcus mutans, and Enterococcus faecalis can be hampered with AgNPs resin cements [33]. Furthermore, the applications of nanoparticles in various forms either combined with polymers or coated onto biomaterial surfaces was found to be having better antimicrobial properties in the oral cavity [34]. In addition to Ag Nps, zinc oxide, magnesium oxide, and silica particles have also been investigated and found them exhibiting effective antimicrobial properties [35, 36].
Although a limited number of studies have examined the antibacterial properties of resin cement when combined with the nanoparticles. A comprehensive investigation of this subject has not yet been undertaken. Resins susceptible to bacterial colonization and infection are commonly used in restorative and prosthetic dentistry. These nanoparticles have unique antibacterial properties. Understanding the antibacterial properties of resin cements, especially those containing nanoparticles, will help in selecting the materials that can effectively prevent bacterial growth and reduce the risk of post-treatment infections. Future research should focus on optimizing the usage, concentration and the combinations of those nanoparticles that can significantly enhance the antibacterial properties of resin cements. Comparison of resin cements with and without nanoparticles allows dental professionals to make informed decisions when selecting materials for various dental applications.
Systematic reviews provide a comprehensive analysis of existing scientific literature. This review contributes to evidence-based dentistry by summarizing and evaluating available research on the antibacterial properties of resin cements with and without nanoparticles. Moreover, this could potentially facilitate future investigations, advancements and utilization of nanotechnology to enhance the effectiveness and efficiency of resin cements.
The hypothesis for the research is following:
“Resin cement containing nanoparticles exhibits enhanced antibacterial properties compared to resin cement without nanoparticles when used in dental applications.”
This hypothesis is based on the premise that the incorporation of nanoparticles into resin cement has the potential to augment its antibacterial characteristics, leading to improved resistance against bacterial colonization and reduced risk of post-treatment infections. By comparing the antibacterial activity of resin cements with and without nanoparticles, it is expected that the former will demonstrate superior performance in inhibiting bacterial growth and preventing secondary caries.
Materials and methods
The authors ensured that they complied with all the PRISMA recommendations for reporting systematic reviews and meta-analyses [37]. The protocol used for this systematic review was the registered International Platform of Registered Systematic Review and Meta-Analysis Protocols (INPLASY) (2023100035).
Research question
What is the comparative analysis of the antibacterial properties of resin cements with and without the incorporation of nanoparticles?
Eligibility criteria
The studies included in our analysis were selected based on their adherence to the PICOS criteria, which included the following elements: population, intervention, control, outcomes, and research design.
The PICOS questions for this study are as follows:
Population: Resin cement.
Intervention: Use of nanoparticles.
Control: Resin cement without nanoparticles.
Outcomes: Antibacterial properties.
Study Design: Comparative studies (both randomized and non-randomized) were conducted in vivo and in vitro. Exclusions were made for single-arm studies, conference abstracts, and studies using composite resin or resin-modified glass ionomer cement as well as those with commercial control groups.
Inclusion Criteria:
Population: Studies focusing on resin cement.
Intervention: Studies that utilized nanoparticles in conjunction with resin cement.
Control: Studies that included a control group that used resin cement without nanoparticles.
Outcomes: Articles that provide a comprehensive analysis of outcomes relevant to the research aims.
Research design: Comparative studies incorporating both randomized and non-randomized methodologies were conducted either in vivo or in vitro.
Exclusion criteria:
Single-arm studies: Studies with only one group or arm without a comparative design.
Conference publications: Papers published solely as conference abstracts or proceedings.
Utilization of composite resin or resin-modified glass ionomer cement: Studies that involve the use of these materials are not within the scope of this analysis.
Commercial control groups: Studies that included control groups consisting of commercially available products or materials.
Information source
A comprehensive search was conducted in PubMed and Scopus databases from their inception until September 12, 2023, using the following search strategy: (cement* AND resin*) AND (nanoparticle* OR nanotechnology) AND (antibact* OR anti-bact* OR antimicro* OR anti-micro* OR antibiofilm* OR anti-biofilm* OR antiinfect* OR anti-infect* OR bactericidal* OR bacteriostatic*). No language or publication type filters were applied. The reference lists of selected articles were also reviewed for additional relevant studies.
Selection process
Endnote software was used to consolidate and merge all individual information. The data were converted into an Excel spreadsheet and submitted in two distinct steps to identify the studies that met the eligibility criteria. Titles and abstracts were independently screened by two reviewers for potential inclusion. Full texts of potentially eligible studies were then independently assessed for eligibility. Discrepancies were resolved through discussion or consultation with a third reviewer.
Data collection process
In this systematic review, the primary author played a crucial role in developing the meticulously organized Excel spreadsheets. These spreadsheets served as valuable tools for reviewing authors to extract relevant data from the included studies. The systematic extraction process involved two researchers who independently collected the data from each study to ensure a thorough and comprehensive approach.
To maintain accuracy and consistency, collaborating researchers engaged in collaborative discourse, discussed, and analyzed the findings obtained from the data extraction process. This collaborative effort helped to minimize errors and enhance the reliability of the extracted data.
The suggested methodologies outlined in the Cochrane Handbook were followed diligently [38]. These methodologies provide a standardized approach for addressing and resolving any discrepancies or missing information. By adhering to these suggested methodologies, the authors maintained the integrity and validity of the data, ensuring that the final findings were based on reliable and accurate information.
Data items (outcomes)
The primary focus of this study was to investigate and compare the antibacterial characteristics of resin cements using specific methodologies to assess their effectiveness in preventing bacterial growth. The colony-forming unit (CFU) count, a key measurement, provided valuable information regarding the antibacterial properties of the different resin cements under investigation. Additionally, bacterial metabolic activity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Incorporating these measurements—CFU counts and MTT activity, enabled a comprehensive evaluation of the antibacterial properties of resin cements.
Data items (other variables)
The authors of this study compiled several parameters, including study ID, study title, resin cement type, nanoparticle type, description of the intervention and control, specifics of antibacterial tests, and species of bacteria employed, as well as the findings derived from each investigation.
Effect measures and synthesis methods
In the process of systematic review, findings from the included studies were qualitatively synthesized to assess the overall quality of evidence and to understand the variations in study outcomes. The synthesis focuses on the narrative description of results, considering the reported means, standard deviations (SDs), and the context of each study’s findings within the broader literature. The methodological quality and potential biases of the included studies were evaluated using appropriate quality assessment tools, tailored to the study designs (e.g., randomized control trials, observational studies). This assessment helps to identify the strengths and limitations of the evidence, including any potential sources of variability among study findings. Instead of statistical heterogeneity analysis, a narrative synthesis is conducted to explore differences in study characteristics, populations, interventions, and outcomes to explain variations in the study findings. The aim is to provide a comprehensive overview of the current state of research on the topic, highlighting consistencies and discrepancies across studies, and to identify areas where the evidence is strong or where further research is needed.
Citation chaser methodology
In addition to the comprehensive search conducted in PubMed and Scopus, we employed a citation chasing methodology to further ensure the thoroughness of our literature review. This process involved reviewing the reference lists of all the studies that were identified through our initial database search. The objective was to identify any additional relevant studies that might not have been captured by our search strategy.
However, it is important to note that the citation chasing process did not yield any additional studies beyond those already identified through the database search. This outcome suggests that our initial search strategy was comprehensive and successfully captured all pertinent studies within the scope of our review. This finding reinforces the robustness of our methodology in ensuring a thorough and exhaustive review of the existing literature.
Results
Literature search
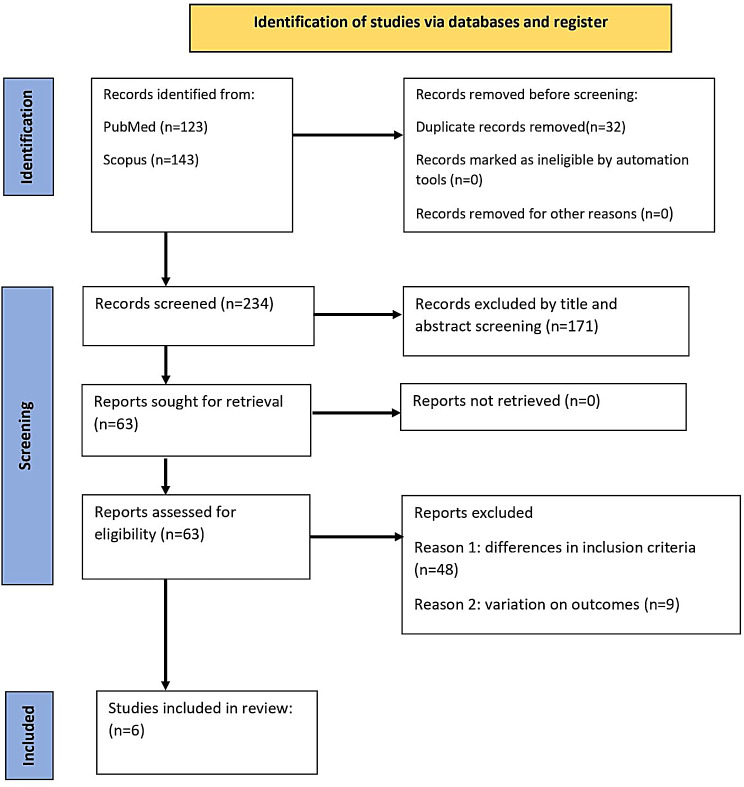
A comprehensive search was conducted during the initial stages of this systematic review, resulting in the identification of 266 relevant records. These records were then subjected to a meticulous screening process that involved evaluating the titles and abstracts to determine their suitability for inclusion in the study. Consequently, 63 records progressed to the subsequent stage of full-text screening, and a more detailed assessment was performed.
Following a rigorous evaluation of the full-text articles, 57 records were excluded for various reasons, such as not meeting the specific inclusion criteria outlined for this study. Ultimately, seven studies met the requirements and were included in the final analysis. To provide a visual representation of the selection process, Fig. 1 presents a flowchart depicting the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The relevance of this information lies in its transparency and methodological rigor. By disclosing the number of records initially identified, the screening process, and ultimately, the inclusion of studies, this systematic review ensures transparency in its methodology and provides readers with a clear understanding of how the final set of studies was determined.
Fig. 1.
PRISMA Flowchart
Study characteristics
The present analysis includes a total of seven comparable in vitro trials. The antibacterial efficacy of resin cement in conjunction with nanoparticles was evaluated by all researchers in relation to its impact on Streptococcus mutans. This systematic review included several types of nanoparticles namely, silver nanoparticles (AgNPs) in four studies [31, 33, 39–41], whereas magnesium oxide nanoparticles (MgONPs) [42], zinc oxide nanoparticles (ZnONPs) [35], and nano-silica with quaternary ammonium salts [36] each in one study. Table 1 summarizes the relevant features of the analyzed studies.
Table 1.
Studies characteristic
| Study ID | Resin | Intervention | Control | Nanoparticles | Test for antibacterial efficacy | Findings |
|---|---|---|---|---|---|---|
| Lim et al. [31] | Light-cure resin cement | Resin cement + 250 ppm NAg | Resin cement + 0 ppm | Silver nanoparticles (NAg) | Antibacterial performance against cariogenic streptococci using the Agar Diffusion Test | The inclusion of silver nanoparticles in resin cement resulted in a notable deceleration of bacterial growth compared to resin cement without silver nanoparticles. |
| Kreve et al. [33] | Dual-cure resin cement |
1- Resin cement + 2.5% β-AgVO3 2- Resin cement + 5% β-AgVO3 |
Resin cement + 0% β-AgVO3 | Semiconductor nanostructured silver vanadate decorated with silver nanoparticles (β-AgVO3) | Antibacterial performance against Staphylococcus aureus, Streptococcus mutans, and Enterococcus faecalis using the Agar Diffusion Test | Antimicrobial activity against Staphylococcus aureus, Streptococcus mutans, and Enterococcus faecalis was observed at both concentrations. |
| Seo et al. [35] | orthodontic resin cement |
a- 95% Resin + ZnO b- 95% Resin + ZnO + HNTs |
100% Resin |
a- Zinc oxide nanoparticles (ZnONPs) b- Zinc oxide (ZnO)-Loaded Halloysite Nanotubes |
Antibacterial performance against S. mutans using MTT metabolic activity | A nanocomposite material used in this research showed antibacterial efficiency against S. mutans while maintaining the mechanical, physical, and chemical properties of orthodontic resin cement. |
| Hu et al. [36] | Self-adhesive resin cement |
a- Resin cement + 10% Nano-silica b- Resin cement + 7.5% Nano-silica c- Resin cement + 5% Nano-silica d- Resin cement + 2.5% Nano-silica |
Resin cement + 0% Nano-silica | Nano-silica with quaternary ammonium salts | Antibacterial performance against S. mutans using colony-forming unit (CFU) counts | Dental plaque microcosm biofilm metabolic activity was significantly reduced by nano-antibacterial inorganic fillers in resin cement at 2.5% or higher. This finding suggests that the resin cement exhibited an effective antibacterial effect. |
| Magalhães et al. [39] | Resin luting cement (Rely X ARC) | Resin cement + 0.05% NAG | RelyX ARC | Silver nanoparticles (NAg) | Antibacterial performance against S. mutans using the Agar Diffusion Test | Vitrebond exhibited bactericidal action both in the absence and presence of silver, with the latter showing a stronger effect. |
| Magalhães et al. [40] | Two Dual-cure resin cement (one conventional “RelyX ARC” and one self-adhesive, RelyX U200) |
a- RelyX ARC with 0.05% NAg b- RelyX ARC with 0.07% Nag c- RelyX U200 with 0.05% NAg d- RelyX U200 with 0.07% NAg |
1- RelyX U200 2- RelyX ARC |
Silver nanoparticles (AgNPs) | Antibacterial performance against S. mutans. | The incorporation of silver nanoparticles (NAg) into resin-luting cements did not inhibit Streptococcus mutans. The materials containing NAg exhibited noticeable alterations in colour and demonstrated increased sorption capabilities compared to the ones without NAg. |
| Wang et al. [42] | Dual-cure resin cement self-etching adhesive |
a- Resin cement + 10% MgONPs b- Resin cement + 7.5% MgONPs c- Resin cement + 5% MgONPs d- Resin cement + 2.5% MgONPs |
Resin cement + 0% MgONPs | Magnesium oxide nanoparticles (MgONPs) | Antibacterial performance against S. mutans using colony-forming unit (CFU) counts and MTT metabolic activity | The incorporation of magnesium oxide nanoparticles (MgONPs) into resin cements has been found to significantly improve the antibacterial properties of these materials, while maintaining their mechanical, bonding, and physicochemical characteristics. In general, the incorporation of magnesium oxide nanoparticles (MgONPs) into resin cements can serve as a viable option for inhibiting cariogenic bacteria. This approach aims to mitigate the occurrence of secondary caries associated with biofilm formation, hence enhancing the long-term effectiveness of cementation. |
Demographics of Study Participants: Given that the included studies were all in vitro, there were no human or animal participants involved. Instead, the studies focused on bacterial strains, primarily Streptococcus mutans, which is commonly associated with dental caries.
Setting: All studies were conducted in controlled laboratory environments, allowing for precise manipulation of experimental conditions and measurement of outcomes.
Sample Sizes: The sample sizes in the studies varied, typically ranging from 10 to 30 samples per experimental group. These sample sizes were sufficient to provide reliable and statistically significant results within each study.
Interventions: The primary intervention across studies was the incorporation of nanoparticles (NPs) into resin cements. The types of nanoparticles used included silver nanoparticles (AgNPs), magnesium oxide nanoparticles (MgONPs), zinc oxide nanoparticles (ZnONPs), and nano-silica with quaternary ammonium salts.
Intervention Comparisons: Each study compared the antibacterial efficacy of NP-modified resin cements against control groups that used unmodified resin cements. The comparisons were designed to isolate the effect of NPs on antibacterial properties.
Risk of Bias: The risk of bias in these studies primarily arose from unblinded outcome assessments and variability in methods used to measure antibacterial efficacy (e.g., CFU counts vs. optical density measurements). Despite these concerns, the overall methodological rigor was maintained, and results were generally consistent across studies.
Effect of interventions on outcomes
Antibacterial Efficacy: All studies reported that NP incorporation significantly reduced bacterial growth, demonstrated by lower CFU counts, reduced biofilm activity (via MTT assays), and larger inhibition zones in agar tests. AgNPs, in particular, showed a strong inhibitory effect on S. mutans.
Mechanisms of Action: NPs exert their antibacterial effects through mechanisms such as generating reactive oxygen species, disrupting cell membranes, and releasing bioactive ions that inhibit bacterial replication.
Variability in Results: While most studies confirmed the antibacterial benefits of NPs, some, like Magalhães et al. [40] found no significant effects using certain measurement methods. This highlights the need for standardized testing protocols and suggests that NP efficacy may vary with concentration, particle size, and bacterial strain.
Qualitative synthesis
Two studies [36, 42] investigated the effects of NPs on CFU counts in resin cements and discovered that NPs dramatically lowered CFU counts compared to resin cements without NPs. Two studies [35, 42] investigated the influence of NPs on MTT metabolic activity and discovered that resin cements containing NPs dramatically reduced biofilm metabolic activity compared to a control group. The agar diffusion test was used in three studies to evaluate the antibacterial performance. Kreve et al. [33]discovered that NPs significantly inhibits Staphylococcus aureus, Streptococcus mutans, and Enterococcus faecalis in a dose dependent manner. Lim et al. [31] discovered that resin cement containing silver nanoparticles considerably reduced bacterial growth compared with resin cement containing no silver nanoparticles. Magalhães et al. [39] revealed that the incorporation of silver NPs enhanced bactericidal activity. Only one study [40] found that NPs had no antibacterial effect, as measured by the optical density of the culture broths.
Discussion
Currently, Resin cement is a widely used luting material for dental applications. Resin cements exhibit superior mechanical and physical qualities compared with their predecessors in the field of luting materials. Nevertheless, the available information indicates that resin cement may exhibit lower efficacy in preventing the formation of caries than conventional luting materials. Nanotechnology is a potential solution, and the addition of NPs to resin cements can improve their antibacterial properties. This study is the first comprehensive evaluation of the antibacterial effectiveness of resin cements with and without nanoparticles (NPs). Research indicates that nanoparticles in resin cement significantly reduce bacterial colony numbers and metabolic activity. Moreover, the current systematic review revealed that the utilization of NPs resulted in the generation of a more extensive zone of inhibition and deceleration of bacterial proliferation in comparison to the control cohort.
Integration and implementation of nanoparticles in dentistry is encouraged due to their broad-spectrum antibacterial impact. Unlike antibiotics, they have an advantage of not developing bacterial resistance despite repeated use [43–45]. Silver NPs and magnesium oxide NPs are the most common NPs known for their antibacterial effects. However, the underlying mechanisms are not fully understood. There are several mechanisms that have been put forward to explain this property. According to a hypothesis, the oxygen changes into reactive oxygen species and hydroxyl radicals which in turn cause structural damage to the bacteria [46, 47]. Another hypothesis states that the silver and magnesium oxide ions are bioactive and when released, they interact with cell membrane structures to prevent the replication of bacteria [46, 48]. Lastly, another hypothesis postulates that the NPs, when attached to the cell membrane, release ions at a very high concentration which are cytotoxic in nature, thus act as anti-bacterial [47].
The seven in vitro studies analyzed offer valuable insights into the types of nanoparticles used and their effects on resin cements. The predominant use of silver nanoparticles (AgNPs) in four out of the seven studies can be attributed to their well-documented broad-spectrum antibacterial properties. AgNPs’ ability to inhibit bacterial growth, as shown by the substantial reduction in colony-forming units (CFU) and bacterial metabolic activity, corroborates the hypothesis that incorporating NPs into resin cements enhances their antibacterial capabilities [49].
Interestingly, the use of magnesium oxide nanoparticles (MgONPs) and zinc oxide nanoparticles (ZnONPs) in resin cements also demonstrated significant antibacterial effects. This suggests that while AgNPs are widely recognized, other NPs such as MgONPs and ZnONPs may offer similar, if not complementary, benefits in enhancing resin cement properties [49]. The diversity in NP types and their consistent antibacterial effects across different studies strengthens the argument for their integration into resin cements for dental applications.
The findings align with a previous systematic review [50], concluding that nanoparticles-modified dental materials exhibit significantly enhanced antibacterial activity compared to untreated materials. However, they did not assess the efficacy of the resin cement modified with NPs, and only assessed the antibacterial effect measured by CFU. In the present systematic review, similar effects were found for resin cement integrated with NPs. Additionally, NPs had a positive effect on MTT metabolic activity.
The qualitative synthesis of the antibacterial efficacy of resin cements containing NPs provides further evidence of their superiority over traditional resin cements. The studies consistently showed that NPs significantly reduced bacterial growth, with silver nanoparticles proving particularly effective. The reduction in CFU counts and the inhibition of biofilm metabolic activity suggest that NPs disrupt bacterial colonization and proliferation, which are essential in the prevention of secondary caries.
However, the variability in the results across different studies, particularly the findings of that showed no significant antibacterial effect of NPs, indicates the complexity of NP interactions with bacterial cells [40]. This could be due to differences in experimental conditions, NP concentrations, or methods of measuring antibacterial efficacy. The non-significant results in some studies highlight the need for standardized protocols to assess the antibacterial properties of NP-modified resin cements effectively.
The higher antibacterial performance of NPs was consistent across all included studies, except that of Magalhães et al. [40]. Magalhães et al. measured the antibacterial performance of silver NPs using the optical density of the broths and found no significant difference between the groups. A similar finding was reported by Wang et al. [42], in which magnesium NPs significantly reduced CFU and MTT, but produced a non-significant effect on the optical density. Wang et al. [42] suggested that MgONPs may directly inactivate adherent bacteria rather than inhibiting plankton development by releasing a high concentration of bioactive material into the culture.
The antibacterial efficacy exhibited a positive correlation with the filler content, whereas the mechanical characteristics demonstrated a negative association, yielding an inverse outcome [51]. It is worth noting that the included studies showed that NPs did not compromise the mechanical, bonding, or physicochemical performance of resin cement. Wang et al. [42] discovered that magnesium NPs had no effect on the shear bond strength, water sorption, or solubility when MgONPs were added at less than 10%. Seo et al. [35] reported similar results, revealing that ZnO NPs exhibit strong antibacterial effects.
The concentration of NPs is one of the most important factors influencing their antibacterial properties. According to Wang et al. [42], the antibacterial effect of MgONPs was concentration-dependent, with the maximum significant drop in CFU counts occurring at 10% MgONPs and no significant effect detected at 2.5% MgONPs. Lim et al. [31] discovered that increasing the concentration of AgNPs resulted in greater suppression of bacteria, as measured by the agar diffusion test. However, Hu et al. [36] discovered that 10% nanosilica and 2.5% nanosilica had similar effects on CFU counts, and Wang et al. [42] found that 10% MgONPs reduced MTT metabolic activity by less than 7.5% and 5%, respectively, indicating a possible capping effect in which the antibacterial effect does not significantly increase beyond certain concentrations. Future studies are needed to confirm the optimal dose at which maximum antibacterial activity is achieved.
Reducing the particle size to the nanoscale causes agglomeration owing to the increased surface/volume ratio [32]. According to Wang et al., [42], MgONPs in resin cements showed a relatively homogeneous distribution; however, dose-dependent particle aggregation was observed. The agglomeration of nanoparticles can have a significant impact on material performance and characteristics, potentially leading to increased fracture susceptibility and decreased material strength. This can be achieved by altering the nanoparticle surface via grafting with inorganic particles or polymers [42].
Most of the included studies assessed the antibacterial properties of resin cement incorporated with nanoparticles against S. mutans. This is attributed to the fact that S. mutans is essential and active in early bacterial colonization and the production of cariogenic plaques [52, 53]. Other types of microbes were also tested. Kreve et al. 2022 [33] reported that resin cement with silver nanoparticles showed antibacterial properties against Staphylococcus aureus, Streptococcus mutans, and Enterococcus faecalis. Further studies are needed to test the effects of NPs incorporated into resin cement on other types of organisms.
Antibacterial activity is not exclusive to the NPs mentioned in the included studies, as other nanoparticles have been reported to demonstrate antimicrobial activity, such as gold, copper, iron, titanium, and cerium NPs [54]. However, silver NPs were used in most of the included studies, which could be attributed to the broad spectrum of their antibacterial properties [55].
The findings of this study have substantial implications for the field. Secondary caries at the interface between dental restorations and teeth, along with subsequent acid attacks, pose a significant challenge to the durability of restorations. This review revealed that the incorporation of nanoparticles (NPs) effectively decreases bacterial colonization and metabolic activity. By integrating NPs into resin cement, we can harness the material’s inherent advantages in terms of chemical and physical properties while simultaneously augmenting its antibacterial effects. Consequently, NP-modified resin cement has emerged as a viable solution for mitigating biofilm-associated secondary caries and enhancing cementation durability. Further research involving in vivo experiments and randomized clinical trials should be conducted to substantiate these findings.
Strengths and limitations
This analysis represents the first meta-analysis and systematic review evaluating the efficacy of NPs in addition to resin cements, including trials regardless of language. However, this study had several limitations. First, the pooled analysis showed significant heterogeneity, which could be attributed to the different types and concentrations of nanoparticles used as well as the different incorporation methods that were applied. However, we used a random-effects model to produce more robust results. Moreover, the absence of sufficient data hindered our ability to conduct subgroup analyses to examine the impact of various types and quantities of nanoparticles. The studies in this review varied in numerous ways, including the incubation period, sample handling techniques, and the specific types of resin cement employed.
Conclusions
The analysis demonstrates a substantial enhancement in the antibacterial properties of resin cement through the incorporation of nanoparticles (NPs), without compromising the mechanical, physical, and chemical characteristic of the resin cement. However, to validate these findings and assess the efficacy of NP-modified resin cements for the prevention of secondary caries, it is crucial to conduct future clinical trials. Moreover, owing to the significant heterogeneity observed in the results, additional research is necessary to establish a standardized protocol for the selection of NP type, optimal concentration, and appropriate method of incorporation.
Acknowledgements
Open Access funding provided by Qatar National Library. All the authors are thankful to King Khalid University, Saudi Arabia, for the financial Support.
Author contributions
Conceptualization, R.S.; S.K.V.; S.A.; D.S.; K.C.S.; methodology, R.S.; S.K.V.; S.A.Q.; S.A.; D.S.; K.C.S.; software, R.S.; S.A.Q.; S.A.B.; S.A.; K.C.S.; validation, R.S.; S.K.V.; M.A.K.; S.A.Q.; K.C.S.; formal analysis, R.S.; D.S.; K.C.S.; writ-ing—original draft preparation, R.S.; S.K.V.; S.A.; D.S.; K.C.S.; writ-ing—review and editing, R.S.; S.K.V.; M.A.K.; S.A.Q.; S.A.B.; S.A.; D.S.; K.C.S.; supervision, R.S.; funding acquisition, S.A.B.; and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia, for supporting the present research study through [GRP/246/44].
Data availability
The data will be available on reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sukumaran Anil, Email: asukumaran1@hamad.qa.
Deepti Shrivastava, Email: sdeepti20@gmail.com.
Kumar Chandan Srivastava, Email: drkcs.omr@gmail.com.
References
- 1.Alam MK, Srivastava KC, Khamis MF, Husein A, Editorial. Recent advancements in the dental biomaterials applied in various diagnostic, restorative, regenerative, and therapeutic procedures. Front Bioeng Biotechnol. 2023;10. [DOI] [PMC free article] [PubMed]
- 2.Materials for Adhesion and Luting. In. Craig’s restorative Dental materials. Elsevier; 2019. pp. 273–94.
- 3.Bilgrami A, Alam MK, Qazi F, ur R, Maqsood A, Basha S, Ahmed N, et al. An In-Vitro Evaluation of Microleakage in Resin-based restorative materials at different time intervals. Polym (Basel). 2022;14:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon JF, Darnell LA. Considerations for proper selection of dental cements. Compend Contin Educ Dent. 2012;33(32):28–30. quiz 36, 38. [PubMed] [Google Scholar]
- 5.Burgess JO, Ghuman T, Cakir D, Swift Jr. EJ. SELF-ADHESIVE RESIN CEMENTS. J Esthetic Restor Dentistry. 2010;22:412–9. [DOI] [PubMed] [Google Scholar]
- 6.Janani K, Teja KV, Sandhya R, Alam MK, Al-Qaisi RK, Shrivastava D, et al. Monomer Elution from three Resin composites at two different time interval using high performance liquid Chromatography—An In-Vitro Study. Polym (Basel). 2021;13:4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaddamanu SK, Vyas R, Bavabeedu SS, Arora S, Badiyani BK, Kumar A. In vitro results of scanning technique on assessing cement thickness and interfacial nanoleakage of luted CAD/CAM-Fabricated Fiber posts. J Pharm Bioallied Sci. 2021;13(Suppl 1):S676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung GK-H, Wong AW-Y, Chu C-H, Yu OY. Update on Dental Luting materials. Dent J (Basel). 2022;10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengar EV, Mulay S, Beri L, Gupta A, Almohareb T, Binalrimal S, et al. Comparative evaluation of Microleakage of Flowable Composite Resin using Etch and Rinse, Self-Etch Adhesive systems, and Self-Adhesive Flowable Composite Resin in Class V cavities: Confocal Laser Microscopic Study. Materials. 2022;15:4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addugala H, Venugopal VN, Rengasamy S, Yadalam PK, Albar NH, Alamoudi A, et al. Marginal and Internal Gap of Metal Copings fabricated using three types of Resin patterns with subtractive and additive technology: an in Vitro comparison. Materials. 2022;15:6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvaraj H, Krithikadatta J, Shrivastava D, Onazi MA, Al, Algarni HA, Munaga S, et al. Systematic review fracture resistance of endodontically treated posterior teeth restored with fiber reinforced composites- a systematic review. BMC Oral Health. 2023;23:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talluri S, Vaddamanu S, Apparaju V, Vyas R, Ahuja S, Kanji M. Evaluating cortico-cancellous ratio using virtual implant planning and its relation with immediate and long-term stability of a dental implant- A CBCT-assisted prospective observational clinical study. Niger J Clin Pract. 2019;22:982. [DOI] [PubMed] [Google Scholar]
- 13.Vaddamanu S, Vyas R, Kavita K, Sushma R, Rani Rp, Dixit A, et al. An in vitro study to compare dental laser with other treatment modalities on biofilm ablation from implant and tooth surfaces. J Pharm Bioallied Sci. 2022;14:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanweer N, Qazi F-U-R, Das G, Bilgrami A, Basha S, Ahmed N, et al. Effect of Erosive agents on Surface characteristics of Nano-Fluorapatite Ceramic: an In-Vitro Study. Molecules. 2022;27:4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gürdal I, Atay A, Eichberger M, Cal E, Üsümez A, Stawarczyk B. Color change of CAD-CAM materials and composite resin cements after thermocycling. J Prosthet Dent. 2018;120:546–52. [DOI] [PubMed] [Google Scholar]
- 16.Wiedenmann F, Becker F, Eichberger M, Stawarczyk B. Measuring the polymerization stress of self-adhesive resin composite cements by crack propagation. Clin Oral Investig. 2021;25:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AKEHASHI S, TAKAHASHI R, NIKAIDO T, BURROW MF. Enhancement of dentin bond strength of resin cement using new resin coating materials. Dent Mater J. 2019;38:955–62. [DOI] [PubMed] [Google Scholar]
- 18.El-Deeb H, Mobarak E. Repair bond strength of high-viscosity glass-ionomer cements using Resin Composite Bonded with Light- and self-cured Adhesive systems. Oper Dent. 2021;46:45–53. [DOI] [PubMed] [Google Scholar]
- 19.Mishra S, Chaturvedi S, Ali M, Pandey KK, Alqahtani NM, Alfarsi MA, et al. Dimensional Stability of Light-activated urethane dimethacrylate denture base resins. Polym (Basel). 2023;15:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam LE, Chan GP, Yim D. In vitro caries inhibition effects by conventional and resin-modified glass-ionomer restorations. Oper Dent. 1997;22:4–14. [PubMed] [Google Scholar]
- 21.Nagamine M, Itota T, Torii Y, Irie M, Staninec M, Inoue K. Effect of resin-modified glass ionomer cements on secondary caries. Am J Dent. 1997;10:173–8. [PubMed] [Google Scholar]
- 22.Eshed M, Lellouche J, Banin E, Gedanken A. MgF2 nanoparticle-coated teeth inhibit Streptococcus mutans biofilm formation on a tooth model. J Mater Chem B. 2013;1:3985. [DOI] [PubMed] [Google Scholar]
- 23.Lal A, Alam MK, Ahmed N, Maqsood A, Al-Qaisi RK, Shrivastava D, et al. Nano Drug Delivery Platforms for Dental Application: infection control and TMJ Management—A. Rev Polym (Basel). 2021;13:4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jose J, Teja KV, Janani K, Alam MK, Khattak O, Salloum MG, et al. Preparation of a Novel Nanocomposite and its antibacterial effectiveness against Enterococcus faecalis—An in vitro evaluation. Polym (Basel). 2022;14:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesh VS, Venkatesh KV, Sihivahanan D, Yadalam PK, Shrivastava D, Srivastava KC. Effect of microbubble as local drug delivery system in endodontic management - an In-Vitro study. Saudi Dent J. 2024. 10.1016/j.sdentj.2024.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sodagar A, Akhoundi MSA, Bahador A, Jalali YF, Behzadi Z, Elhaminejad F, et al. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dent Press J Orthod. 2017;22:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia PPNS, Cardia MFB, Francisconi RS, Dovigo LN, Spolidório DMP, de Souza Rastelli AN, et al. Antibacterial activity of glass ionomer cement modified by zinc oxide nanoparticles. Microsc Res Tech. 2017;80:456–61. [DOI] [PubMed] [Google Scholar]
- 28.Teja KV, Janani K, Srivastava KC, Shrivastava D, Natoli V, Di Blasio M, et al. Comparative evaluation of antimicrobial efficacy of different combinations of calcium hydroxide against Enterococcus faecalis. BMC Oral Health. 2023;23:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sairaman S, Nivedhitha MS, Shrivastava D, Al Onazi MA, Algarni HA, Mustafa M, et al. Biocompatibility and antioxidant activity of a novel carrageenan based injectable hydrogel scaffold incorporated with Cissus quadrangularis: an in vitro study. BMC Oral Health. 2022;22:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janani K, Teja K, Alam M, Shrivastava D, Iqbal A, Khattak O, et al. Efficacy of Oregano essential oil extract in the inhibition of bacterial lipopolysaccharide (LPS)-Induced Osteoclastogenesis using RAW 264.7 murine macrophage cell Line—An In-Vitro Study. Separations. 2021;8:240. [Google Scholar]
- 31.Lim BS, Ann SJ, Park SY. The effect of nanoparticles on the translucency and antimicrobial activities of experimental resin cements. In: Tech Proc 2012 NSTI Nanotechnol Conf Expo, NSTI-Nanotech 2012. 2012. pp. 113–6.
- 32.Song W, Ge S. Application of Antimicrobial nanoparticles in Dentistry. Molecules. 2019;24:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreve S, Botelho AL, Lima da Costa Valente M, Bachmann L, Schiavon MA, Dos Reis AC. Incorporation of a β-AgVO3 Semiconductor in Resin Cement: evaluation of Mechanical properties and Antibacterial Efficacy. J Adhes Dent. 2022;24:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saafan A, Zaazou MH, Sallam MK, Mosallam O, El Danaf HA. Assessment of Photodynamic Therapy and nanoparticles effects on Caries models. Open Access Maced J Med Sci. 2018;6:1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo J-H, Kim K-M, Kwon J-S. Antibacterial and Physicochemical Properties of Orthodontic Resin Cement Containing ZnO-Loaded Halloysite Nanotubes. Polym (Basel). 2023;15:2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu G, Zhang X-Y, Zhao J-X, Zhou C-J, Wu J-L. [Development of novel self-adhesive resin cement with antibacterial and self-healing properties]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020;38:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editors. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. [DOI] [PMC free article] [PubMed]
- 39.Magalhães APR, Santos LB, Lopes LG, Estrela CR, de Estrela A, Torres C. Nanosilver Application in Dental cements. ISRN Nanatechnol. 2012;2012:1–6. [Google Scholar]
- 40.Magalhaes A, Moreira F, Alves D, Estrela C, Estrela C, Carriao M et al. Silver nanoparticles in resin luting cements: Antibacterial and physiochemical properties. J Clin Exp Dent. 2016;:0–0. [DOI] [PMC free article] [PubMed]
- 41.Apparaju V, Vaddamanu SK, Mandali BK, Vyas R, Gurumurthy V, Vishwanath S. Does the quality of residual alveolar bone apical to a periodontal lesion beneath the maxillary sinus play a vital role in preventing the extension of periodontal disease to maxillary sinus? A CBCT-assisted retrospective study. Technol Health Care. 2021;29:911–20. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Wu Z, Wang T, Tian J, Zhou Z, Guo D, et al. Antibacterial and physical properties of resin cements containing MgO nanoparticles. J Mech Behav Biomed Mater. 2023;142:105815. [DOI] [PubMed] [Google Scholar]
- 43.Priyadarsini S, Mukherjee S, Mishra M. Nanoparticles used in dentistry: a review. J Oral Biol Craniofac Res. 2018;8:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng L, Weir MD, Xu HHK, Antonucci JM, Lin NJ, Lin-Gibson S, et al. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J Biomed Mater Res B Appl Biomater. 2012;100B:1378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alam MK, Kamal MA, Srivastava KC. Special issue on current concepts and challenges in oral health: implications for the Global Population. Appl Sci. 2023;13:3140. [Google Scholar]
- 46.Saengmee-anupharb S, Srikhirin T, Thaweboon B, Thaweboon S, Amornsakchai T, Dechkunakorn S, et al. Antimicrobial effects of silver zeolite, silver zirconium phosphate silicate and silver zirconium phosphate against oral microorganisms. Asian Pac J Trop Biomed. 2013;3:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahn S-J, Lee S-J, Kook J-K, Lim B-S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–13. [DOI] [PubMed] [Google Scholar]
- 48.Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–8. [DOI] [PubMed] [Google Scholar]
- 49.Róna V, Bencze B, Kelemen K, Végh D, Tóth R, Kói T, et al. Effect of Chitosan on the number of Streptococcus mutans in saliva: a Meta-analysis and systematic review. Int J Mol Sci. 2023;24:15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrando-Magraner E, Bellot-Arcís C, Paredes-Gallardo V, Almerich-Silla JM, García-Sanz V, Fernández-Alonso M, et al. Antibacterial properties of nanoparticles in Dental Restorative materials. A systematic review and Meta-analysis. Med (B Aires). 2020;56:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barot T, Rawtani D, Kulkarni P. Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with enhanced Physico-Mechanical and Biological properties for Dental Applications. J Compos Sci. 2020;4:81. [Google Scholar]
- 52.Kasraei S, Sami L, Hendi S, AliKhani M-Y, Rezaei-Soufi L, Khamverdi Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 2014;39:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Géczi Z, Róth I, Kőhidai Z, Kőhidai L, Mukaddam K, Hermann P, et al. The use of Trojan-horse drug delivery system in managing periodontitis. Int Dent J. 2023;73:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su C, Huang K, Li H-H, Lu Y-G, Zheng D-L. Antibacterial properties of Functionalized Gold nanoparticles and their application in oral Biology. J Nanomater. 2020;2020:1–13. [Google Scholar]
- 55.Cheng L, Zhang K, Weir MD, Melo MAS, Zhou X, Xu HH. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine. 2015;10:627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on reasonable request from the corresponding author.