Abstract
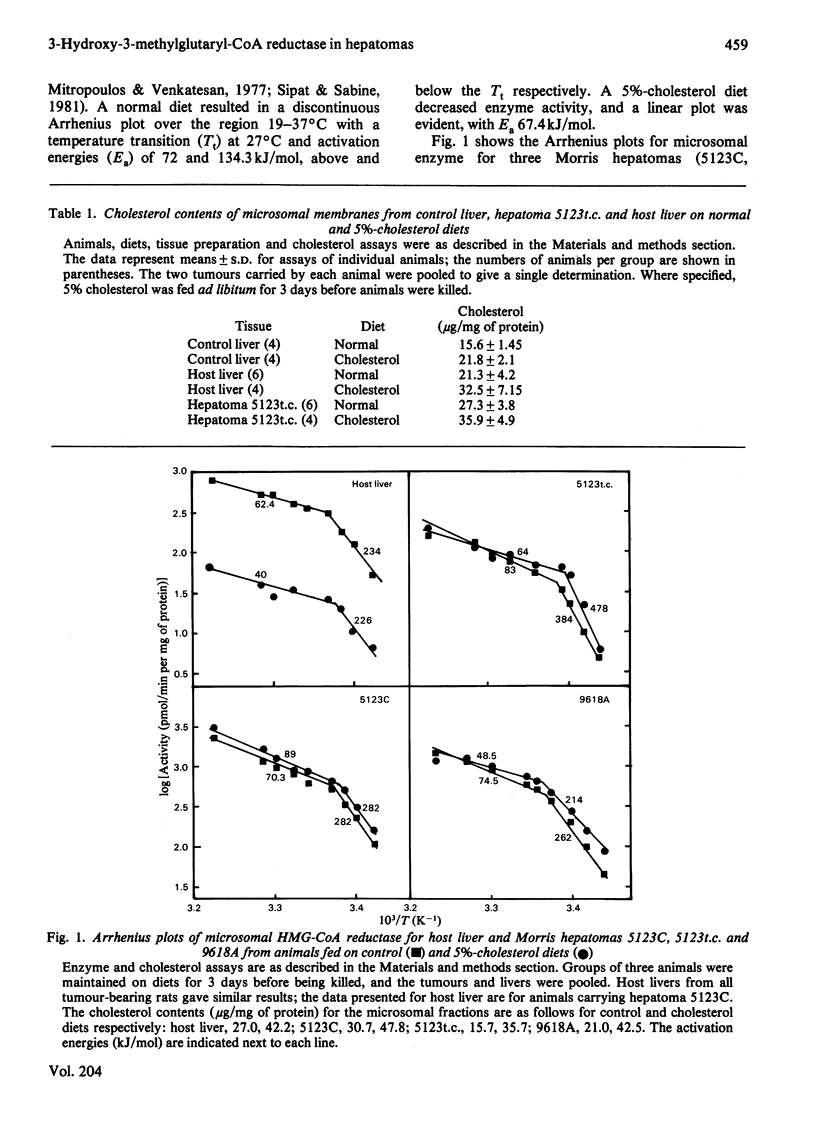
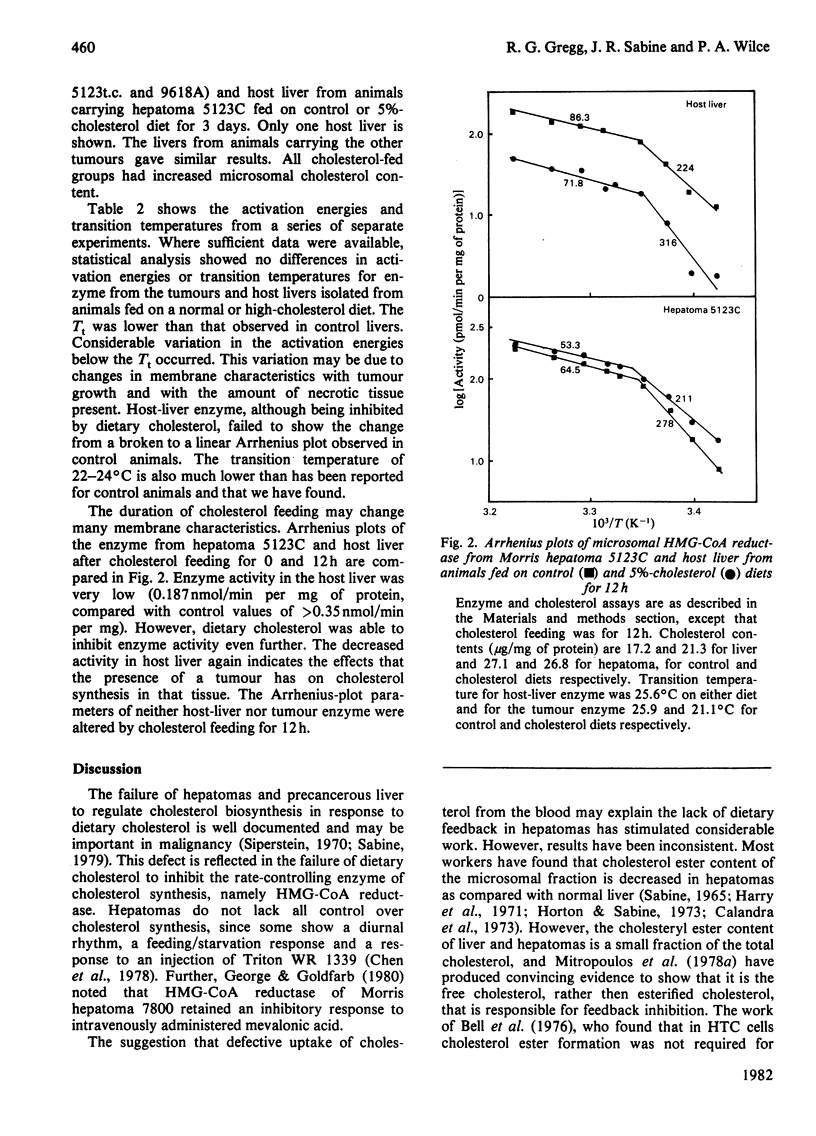
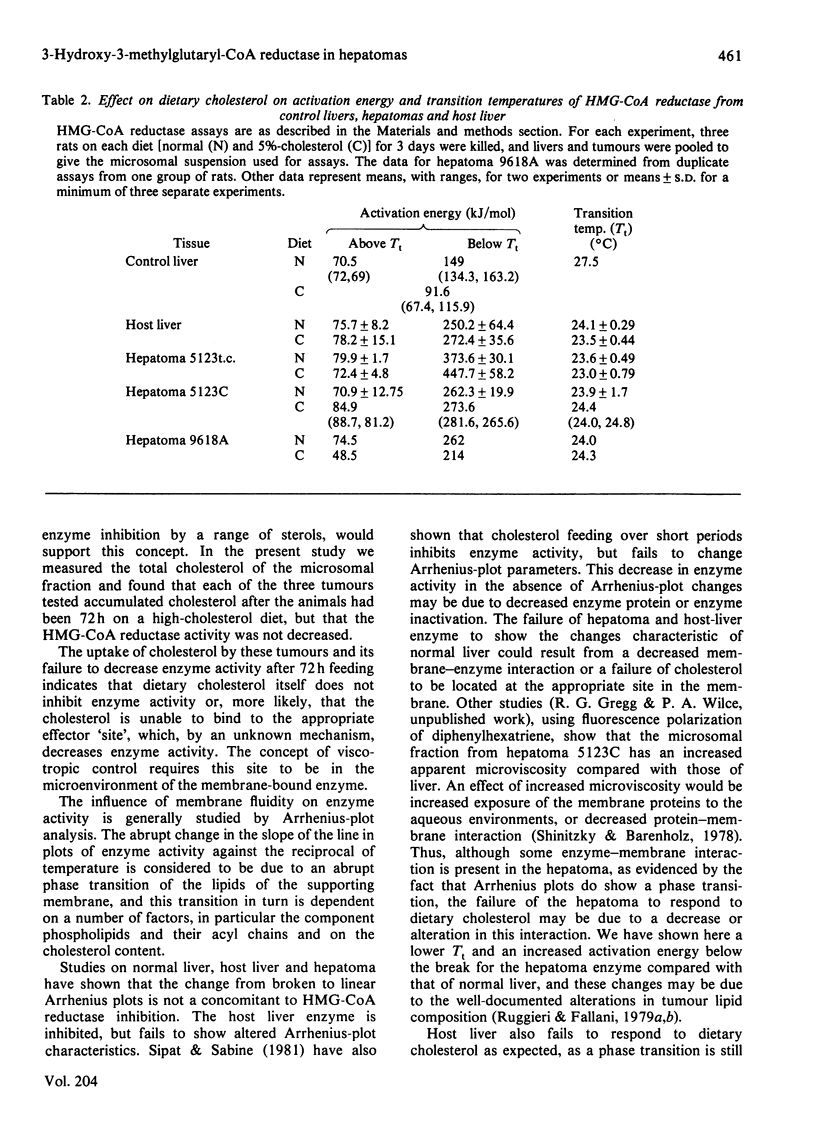
Characteristics of 3-hydroxy-3-methylglutaryl-CoA reductase from normal liver, Morris hepatomas 5123C, 5123t.c. and 9618A, and host liver were studied. Animals were fed on control and 5%-cholesterol diets. Microsomal membranes from all tissues were found to accumulate cholesterol after 3 days on the 5%-cholesterol diet. The enzyme of the tumours showed no feedback inhibition by dietary cholesterol, and that of host liver gave a variable response, whereas that of control liver was constantly inhibited by 90% or more. Arrhenius-plot analysis was conducted on the microsomal enzyme isolated from the various tissues. Control animals showed that the phase transition present at 27 degrees C was removed when animals were fed on 5%-cholesterol diet for 12 h. The hepatomas failed to show this change even after 3 days of 5%-cholesterol diet and a significant increase in microsomal cholesterol. This failure to remove the break in Arrhenius plots also occurred in host liver, even though enzyme inhibition occurred. The reason why hepatomas fail to regulate 3-hydroxy-3-methylglutaryl-CoA reductase activity in response to dietary cholesterol may be a decreased membrane-enzyme interaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. E., Redd W. L., Tormanen C. D., Hardgrave J. E., Scallen T. J. The quantitative assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase: comparison of a thin-layer chromatographic assay with a rapid chloroform extraction assay. J Lipid Res. 1977 May;18(3):408–413. [PubMed] [Google Scholar]
- Bell J. J., Sargeant T. E., Watson J. A. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in hepatoma tissue culture cells by pure cholesterol and several cholesterol derivatives. Evidence supporting two distinct mechanisms.20l. J Biol Chem. 1976 Mar 25;251(6):1745–1758. [PubMed] [Google Scholar]
- Calandra S., Guariento D., Rivasi F. Regulation of cholesterol biosynthesis in rat liver after the administration of N-2-fluorenylacetamide. Lab Invest. 1973 Jun;28(6):723–727. [PubMed] [Google Scholar]
- Chen H. W., Kandutsch A. A., Heiniger H. J. The role of cholesterol in malignancy. Prog Exp Tumor Res. 1978;22:275–316. doi: 10.1159/000401203. [DOI] [PubMed] [Google Scholar]
- George R., Goldfarb S. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in Morris hepatoma 7800 after intravenous injection of mevalonic acid. Cancer Res. 1980 Dec;40(12):4717–4721. [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R., Chen H. W., Cavenee W. K., Kandutsch A. A. Regulation of cholesterol biosynthesis in cultured cells by probable natural precursor sterols. J Biol Chem. 1980 Jan 25;255(2):395–400. [PubMed] [Google Scholar]
- Gibson D. M., Ingebritsen T. S. Reversible modulation of liver hydroxymethylglutaryl CoA reductase. Life Sci. 1978 Dec 31;23(27-28):2649–2664. doi: 10.1016/0024-3205(78)90644-6. [DOI] [PubMed] [Google Scholar]
- Goldfarb S., Pitot H. C. Improved assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1971 Jul;12(4):512–515. [PubMed] [Google Scholar]
- Harry D. S., Morris H. P., McIntyre N. Cholesterol biosynthesis in transplantable hepatomas: evidence for impairment of uptake and storage of dietary cholesterol. J Lipid Res. 1971 May;12(3):313–317. [PubMed] [Google Scholar]
- Horton B. J., Horton J. D., Pitot H. C. Abnormal cholesterol uptake, storage, and synthesis in the livers of 2-acetylaminofluorene-fed rats. Cancer Res. 1973 Jun;33(6):1301–1305. [PubMed] [Google Scholar]
- Horton B. J., Mott G. E., Pitot H. C., Goldarb S. Rapid uptake of dietary cholesterol by hyperplastic liver nodules and primary hepatomas. Cancer Res. 1973 Mar;33(3):460–464. [PubMed] [Google Scholar]
- Horton B. J., Sabine J. R. Metabolic controls in precancerous liver. IV. Loss of feedback control of cholesterol synthesis and impaired cholesterol uptake in ethionine-fed rats. Eur J Cancer. 1973 Jan;9(1):11–17. doi: 10.1016/0014-2964(73)90037-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Venkatesan S., Reeves B. E. On the mechanism for the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, of cholesterol 7alpha-hydroxylase and of acyl-coenzyme A:cholesterol acyltransferase by free cholesterol. Biochim Biophys Acta. 1978 Jul 25;530(1):99–111. doi: 10.1016/0005-2760(78)90130-3. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S. Compartmentation and supply of cholesterol: two important factors in the co-ordinate regulation of hydroxymethyglutary-CoA reductase and cholesterol 7alpha-hydroxylase. Biochem Soc Trans. 1978;6(5):878–883. doi: 10.1042/bst0060878. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S. On the mechanism of regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and of acyl coenzyme A:cholesterol acyltransferase by dietary fat. Biochim Biophys Acta. 1980 Aug 11;619(2):247–257. doi: 10.1016/0005-2760(80)90073-9. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S. The influence of cholesterol on the activity, on the isothermic kinetics and on the temperature-induced kinetics of 3-hydroxy-3-methylglutaryl coenzyme a reductase. Biochim Biophys Acta. 1977 Oct 24;489(1):126–142. doi: 10.1016/0005-2760(77)90239-9. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Ruggieri S., Fallani A. Lipid composition of Morris hepatoma 5123c, and of livers and blood plasma from host and normal rats. Lipids. 1979 Sep;14(9):781–788. doi: 10.1007/BF02533516. [DOI] [PubMed] [Google Scholar]
- Ruggieri S., Fallani A. Lipid composition of Yoshida ascites hepatoma and of livers and blood plasma from host and normal rats. Lipids. 1979 Apr;14(4):323–333. doi: 10.1007/BF02533415. [DOI] [PubMed] [Google Scholar]
- Sabine J. R. Control of cholesterol synthesis in hepatomas: the effect of bile salts. Biochim Biophys Acta. 1969 Apr 29;176(3):600–604. doi: 10.1016/0005-2760(69)90226-4. [DOI] [PubMed] [Google Scholar]
- Sabine J. R., James M. J. The intracellular mechanism responsible for dietary feedback control of cholesterol synthesis. Life Sci. 1976 Jun 1;18(11):1185–1192. doi: 10.1016/0024-3205(76)90191-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Sipat A. B., Sabine J. R. Membrane-mediated control of hepatic beta-hydroxy-beta-methylglutaryl-coenzyme A reductase. Biochem J. 1981 Mar 15;194(3):889–893. doi: 10.1042/bj1940889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solow E. B., Freeman L. W. A fluorometric ferric chloride method for determining cholesterol in cerebrospinal fluid and serum. Clin Chem. 1970 Jun;16(6):472–476. [PubMed] [Google Scholar]


