Abstract
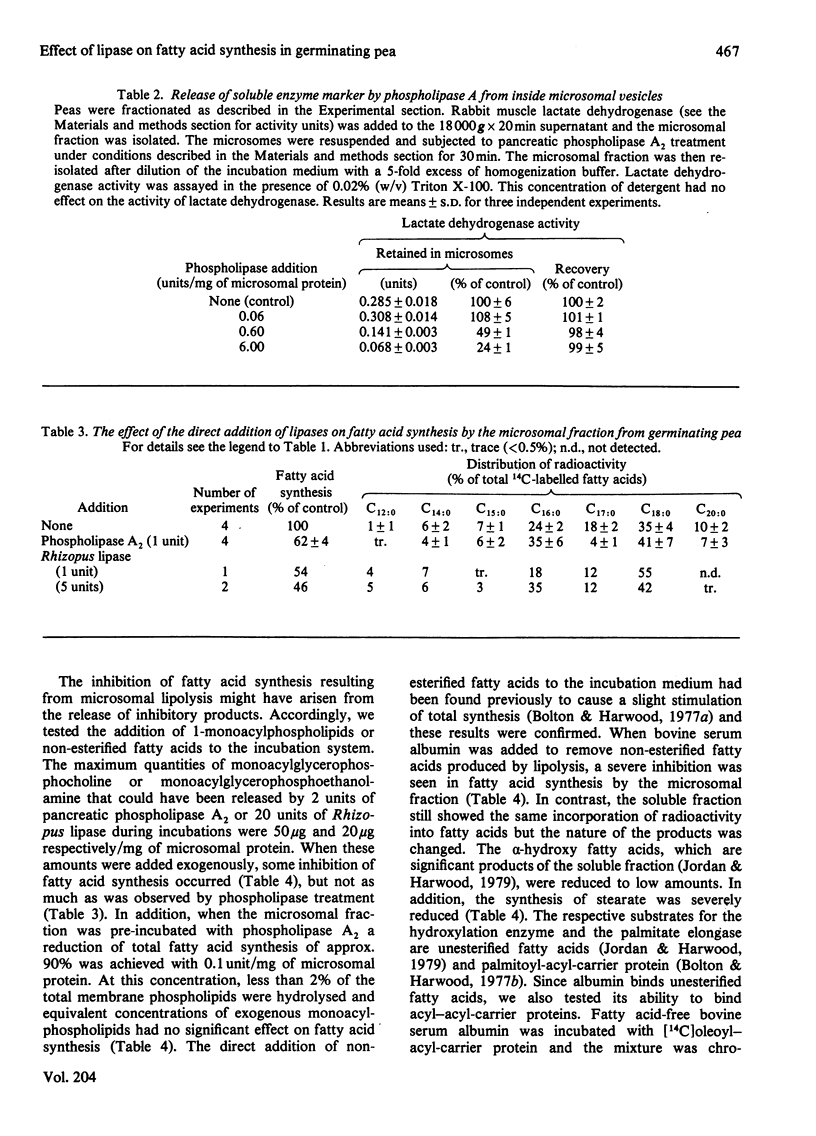
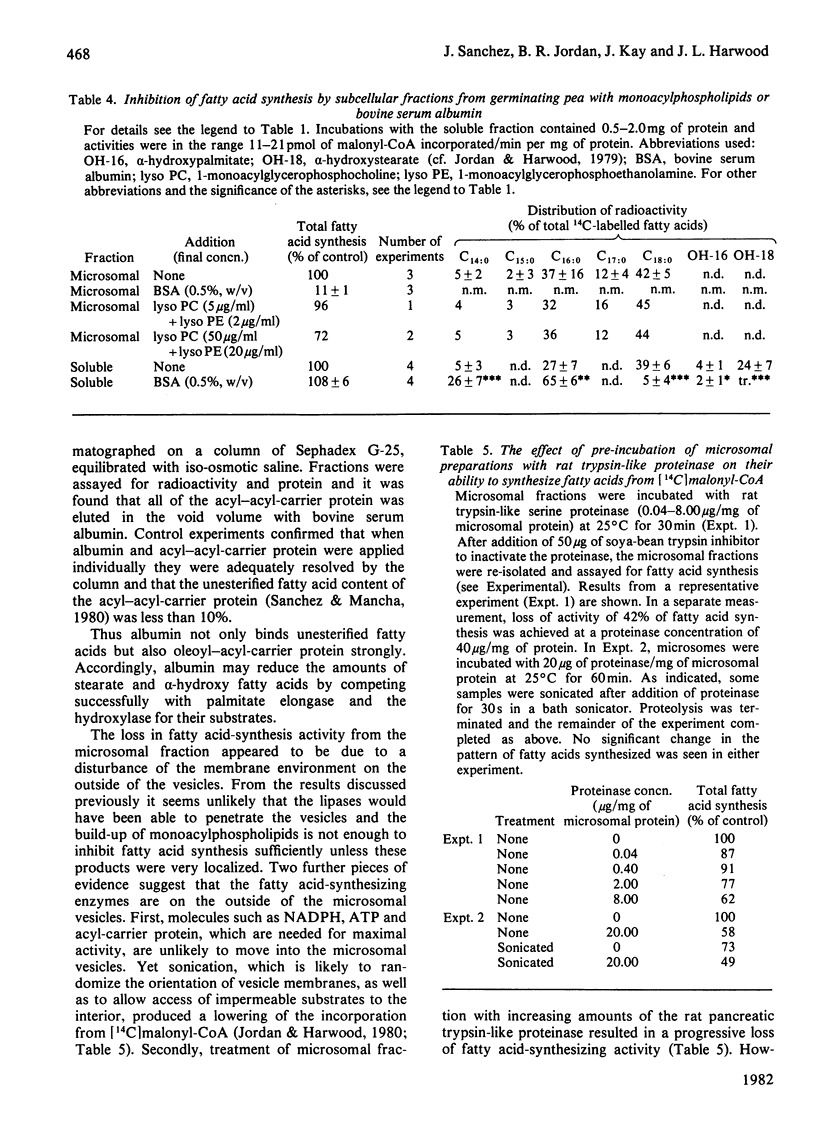
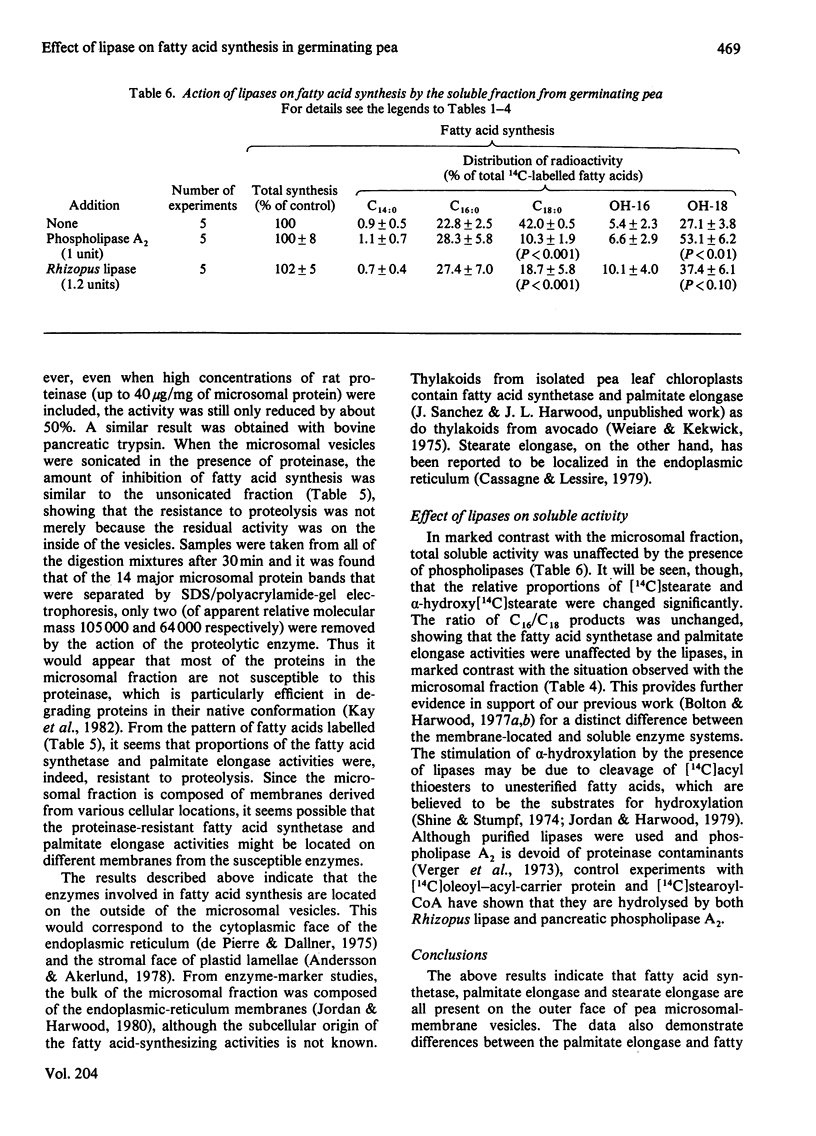
1. The effect of exogenous lipases on fatty acid synthesis from [14C]malonyl-CoA by the microsomal and soluble fractions from germinating peas was studied. 2. Addition of phospholipase A2 or the lipase from Rhizopus arrhizus had no effect on total fatty acid synthesis by the soluble fraction but caused severe inhibition of that by the microsomal fraction. 3. The addition of enzymes with phospholipase activity particularly inhibited the microsomal stearate elongase. 4. Control studies indicated that the phospholipase-induced inhibition of fatty acid synthesis was due to the location of fatty acid synthetase, palmitate elongase and stearate elongase on the outside of the microsomal vesicles. 5. Experiments with a trypsin-like proteinase showed that approximately half the microsomal fatty acid synthesis was resistant to proteolysis. 6. Although addition of exogenous phospholipases had no effect on total fatty acid synthesis by the soluble fraction, it did increase alpha-hydroxylation of newly-formed palmitate and stearate. 7. The results provide further evidence for differences between the soluble and particulate fatty acid synthetase and palmitate elongase activities of germinating pea.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Akerlund H. E. Inside-out membrane vesicles isolated from spinach thylakoids. Biochim Biophys Acta. 1978 Sep 7;503(3):462–472. doi: 10.1016/0005-2728(78)90145-7. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R., Weinstein J. N., Sharrow S. O., Henkart P. Liposome--lymphocyte interaction: saturable sites for transfer and intracellular release of liposome contents. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5603–5607. doi: 10.1073/pnas.74.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton P., Harwood J. L. Fatty acid biosynthesis by a particulate preparation from germinating pea. Biochem J. 1977 Nov 15;168(2):261–269. doi: 10.1042/bj1680261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton P., Harwood J. L. Some characteristics of soluble fatty acid synthesis in germinating pea seeds. Biochim Biophys Acta. 1977 Oct 24;489(1):15–24. doi: 10.1016/0005-2760(77)90227-2. [DOI] [PubMed] [Google Scholar]
- Bourre J. M., Paturneau-Jouas M. Y., Daudu O. L., Baumann N. A. Lignoceric acid biosynthesis in the developing brain. Activities of mitochondrial acetyl-CoA-dependent synthesis and microsomal malonyl-CoA chain-elongating system in relation to myelination. Comparison between normal mouse and dysmyelinating mutants (quaking and jimpy). Eur J Biochem. 1977 Jan 3;72(1):41–47. doi: 10.1111/j.1432-1033.1977.tb11222.x. [DOI] [PubMed] [Google Scholar]
- Cassagne C., Lessire R. Biosynthesis of saturated very long chain fatty acids by purified membrane fractions from leek epidermal cells. Arch Biochem Biophys. 1978 Nov;191(1):146–152. doi: 10.1016/0003-9861(78)90076-0. [DOI] [PubMed] [Google Scholar]
- Cheesbrough T. M., Moore T. S. Transverse Distribution of Phospholipids in Organelle Membranes from Ricinus communis L. var. Hale Endosperm: MITOCHONDRIA AND GLYOXYSOMES. Plant Physiol. 1980 Jun;65(6):1076–1080. doi: 10.1104/pp.65.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger B. F., Vratsanos S. M., Wassermann N., Cooper A. G. A chemical investigation of the active center of pepsin. Biochem Biophys Res Commun. 1966 May 3;23(3):243–245. doi: 10.1016/0006-291x(66)90535-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gan-Elepano M., Mead J. F. The function of phospholipase A2 in the metabolism of membrane lipids. Biochem Biophys Res Commun. 1978 Jul 14;83(1):247–251. doi: 10.1016/0006-291x(78)90423-0. [DOI] [PubMed] [Google Scholar]
- Harwood J. L. The synthesis of acyl lipids in plant tissues. Prog Lipid Res. 1979;18(2):55–86. doi: 10.1016/0163-7827(79)90006-7. [DOI] [PubMed] [Google Scholar]
- Jaworski J. G., Goldschmidt E. E., Stumpf P. K. Fat metabolism in higher plants. Properties of the palmityl acyl carrier protein: stearyl acyl carrier protein elongation system in maturing safflower seed extracts. Arch Biochem Biophys. 1974 Aug;163(2):769–776. doi: 10.1016/0003-9861(74)90539-6. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Harwood J. L. Fatty acid elongation by a particulate fraction from germinating pea. Biochem J. 1980 Dec 1;191(3):791–797. doi: 10.1042/bj1910791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R., Harwood J. L. alpha-Hydroxylation of newly synthesised fatty acids by a soluble fraction from germinating pea. Biochim Biophys Acta. 1979 Apr 27;573(1):218–221. doi: 10.1016/0005-2760(79)90190-5. [DOI] [PubMed] [Google Scholar]
- Kay J., Siemankowski R. F., Siemankowski L. M., Goll D. E. Degradation of smooth-muscle myosin by trypsin-like serine proteinases. Biochem J. 1982 Feb 1;201(2):267–278. doi: 10.1042/bj2010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E., Buckner J. S. Chain elongation of fatty acids by cell-free extracts of epidermis from pea leaves (pisum sativum). Biochem Biophys Res Commun. 1972 Jan 31;46(2):801–807. doi: 10.1016/s0006-291x(72)80212-2. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Shoji S., Funakoshi T., Ueki H. Carboxypeptidase CN. II. Stability and some chemical and kinetic properties. J Biochem. 1974 Aug;76(2):375–384. doi: 10.1093/oxfordjournals.jbchem.a130579. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macey M. J., Stumpf P. K. Fat Metabolism in Higher Plants XXXVI: Long Chain Fatty Acid Synthesis in Germinating Peas. Plant Physiol. 1968 Oct;43(10):1637–1647. doi: 10.1104/pp.43.10.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PESCE A., MCKAY R. H., STOLZENBACH F., CAHN R. D., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF LACTIC DEHYDROGENASES. I. PROPERTIES OF THE CRYSTALLINE BEEF AND CHICKEN ENZYMES. J Biol Chem. 1964 Jun;239:1753–1761. [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Mincey T. C., Dennis E. A. Chemical modification of the histidine residue in phospholipase A2 (Naja naja naja). A case of half-site reactivity. J Biol Chem. 1977 Apr 10;252(7):2405–2411. [PubMed] [Google Scholar]
- SAUER F., PUGH E. L., WAKIL S. J., DELANEY R., HILL R. L. 2-MERCAPTOETHYLAMINE AND BETA-ALANINE AS COMPONENTS OF ACYL CARRIER PROTEIN. Proc Natl Acad Sci U S A. 1964 Dec;52:1360–1366. doi: 10.1073/pnas.52.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]
- Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Recent studies on plant alpha-oxidation systems. Arch Biochem Biophys. 1974 May;162(1):147–157. doi: 10.1016/0003-9861(74)90113-1. [DOI] [PubMed] [Google Scholar]
- Sundler R., Alberts A. W., Vagelos P. R. Phospholipases as probes for membrane sideness. Selective analysis of the outer monolayer of asymmetric bilayer vesicles. J Biol Chem. 1978 Aug 10;253(15):5299–5304. [PubMed] [Google Scholar]
- Verger R., Mieras M. C., de Haas G. H. Action of phospholipase A at interfaces. J Biol Chem. 1973 Jun 10;248(11):4023–4034. [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas G. H., Postema N. M., Nieuwenhuizen W., van Deenen L. L. Purification and properties of phospholipase A from porcine pancreas. Biochim Biophys Acta. 1968 Apr 24;159(1):103–117. doi: 10.1016/0005-2744(68)90248-9. [DOI] [PubMed] [Google Scholar]


