Abstract
Background
Childhood trauma (CT) is a major risk factor for psychiatric disorders. Emotional and cognitive functions are often affected in many psychiatric conditions, and these functions are mediated by the limbic system. However, previous research has primarily focused on patient populations. Therefore, we aim to examine the impact of CT on the limbic brain structure in healthy individuals.
Methods
We enrolled 48 individuals in health, evenly split into two groups: 24 healthy participants with CT (HP-CT) and 24 healthy participants without CT (HP-nCT). They underwent scale assessments and MRI data acquisition. Comparisons between the two groups were performed after subcortical subregion volume segmentation using FreeSufer. Lastly, we examined correlations between volume changes and scale scores.
Results
We found that HP-CT group had smaller volumes in several subregions of the hippocampus, amygdala, and cortical limbic structures, including the subiculum (Sub) head and body, cornu ammonis (CA)1 head, molecular layer (ML) head, granule cell layer of the dentate gyrus (GC-ML-DG) body, CA4 body, fimbria, hippocampus-amygdala transition area (HATA), whole hippocampus head and body, whole hippocampus, basal nucleus (Ba), accessory basal nucleus (AB), cortico-amygdaloid transition area (CAT), paralaminar nucleus (PL) of the left hemisphere; and hippocampal tail, presubiculum (PreSub) body, and basal forebrain of the right hemisphere. Volume changes in the CA4 body and GC-ML-DG body were correlated with sexual abuse. Changes in the volume of the right basal forebrain were linked to emotional neglect. However, these findings were not significant after correction for multiple comparisons.
Conclusion
CT impacts multiple structures of the limbic system, including the hippocampus, and amygdala. This also suggests that region-specific changes within the limbic system can serve as clinical biomarkers supporting cross-diagnostic psychiatric illnesses.
Keywords: Healthy individual, Childhood trauma, Limbic system, Subregions, Volume, Brain imaging
Introduction
Childhood trauma, a multifaceted issue, has far-reaching consequences for both individuals and society. It’s closely associated with mental disorders, such as depression, suicidal risk, and post-traumatic stress disorder (PTSD), while also affecting cognitive function significantly [1]. CT is currently thought to involve the negative effects of physical, sexual, or emotional abuse or neglect, but understanding of this phenomenon is evolving [2]. Neuroimaging methods allow us to identify changes in neuroanatomical structure and function and reveal their correlation with different types of traumatic events. In this regard, research has documented alterations at the level of cortical and subcortical structures [3]. As core structures of the limbic emotion processing circuit, the amygdala and hippocampus are susceptible to early stress exposure [4]. Previous studies have indicated that changes in amygdala volume, either increasing or decreasing, can serve as neural markers of early life stress, while reduced hippocampal volume is commonly found in clinical populations associated with childhood early stress-related emotional disorders [4].
Individuals who have experienced different types of threatening events during childhood, such as physical or sexual abuse, exhibit confirmed volume changes in the hippocampus and amygdala subregions [5, 6], with some studies suggesting that sexual and physical abuse have the greatest impact on the amygdala subregions among various types of CT [7]. Regarding the subfields of the hippocampus, results tend to indicate that CT survivors generally have smaller hippocampal volumes. The volume changes of the amygdala are inconsistent in the timing of trauma and disease backgrounds. Compared with the control group, the right amygdala of schizophrenia patients with PTSD or a history of trauma is smaller [8, 9]. Compared with the healthy control group, the amygdala volume of participants who suffered trauma and PTSD in childhood was smaller. In contrast, the amygdala volume of the two groups who suffered trauma in adulthood was larger than that of the control group that did not suffer trauma [10]. Inconsistent amygdala volume also suggests the possibility of confounding factors.
In addition to the hippocampus and amygdala, other structures of the limbic system, such as the corpus callosum, anterior cingulate cortex, and hypothalamus, also show decreased volumes associated with trauma exposure [11–13]. Moreover, in terms of functional connectivity, changes have been observed in trauma-exposed individuals across regional activation, bivariate functional connectivity, and network-based connectivity [14]. Trauma may affect cognitive function by affecting the functional connectivity of the anterior hippocampus [15]. Furthermore, increased activation of the amygdala, hippocampus, and ventromedial prefrontal cortex during emotion regulation is associated with higher levels of violence exposure. Enhanced functional connectivity between the amygdala and brainstem is associated with higher levels of violence exposure [16].
Research suggests that different types of CT may have different effects on neurodevelopment, which are related to plasticity and neurogenesis [17]. The reduction in hippocampal volume may be associated with elevated levels of stress-related hormones during early life or direct neurotoxic effects, leading to neuronal remodeling, such as synaptic loss [18, 19]. Some studies indirectly support these hypotheses, observing reduced cortisol responses to psychosocial stressors, increased levels of C-reaction protein (CRP), exaggerated amygdala responses to negative emotional stimuli, and decreased gray matter volume in the hippocampus in participants with or without trauma-related psychiatric diagnoses [20]. Childhood abuse and neglect can impact neurotransmitter systems through multiple pathways, affecting various regions of the brain. These pathways include the hypothalamo-pituitary-adrenal axis and the central noradrenergic-sympathoadrenomedullary stress axes and other neurotransmitter systems [21]. These influences are associated with structural changes in the brain, including alterations in cerebral volumes, corpus callosum and cortical hemispheres, prefrontal cortex and amygdalae, superior temporal gyrus, hippocampus as well as the cerebellar vermis [21].
For healthy populations, we also observe a decrease in gray matter volume in the right middle cingulate gyrus [22]. Our analysis shows a significant reduction in the left hippocampal volume in individuals who have experienced abuse [23]. Given the impact of CT on emotional functioning, previous research has focused more on CT effects within the realm of psychiatric disorders. Moreover, there is a paucity of literature that delves into detailed subregion segmentation to investigate volumetric changes. Therefore, this paper aims to subdivide the limbic system into subregions to explore volume changes in healthy populations, thereby better understanding the mechanisms of CT.
Methods
Participants
This study recruited a total of 48 healthy participants aged 18–33 years, comprising 24 individuals with a history of CT and 24 gender-matched participants without such experiences. CT was defined as experiencing chronic moderate to severe trauma exposure, including abuse and neglect, before the age of 16. Participants were recruited through advertisements from local universities and communities, and grouped based on self-reported CT history. All participants underwent thorough interviews conducted by two trained psychiatrists and were screened for psychiatric disorders according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. General exclusion criteria included: (1) significant physical illness; (2) patients with any other Axis I or Axis II mental disorders after structured clinical interview for DSM-IV (SCID) screening; (3) those with a history of alcohol and drug dependence, or patients undergoing hormonal therapy; (4) a family history of bipolar affective disorder; (5) a history of epilepsy or a family history of epilepsy; (6) a history of head injury or loss of consciousness; (7) pregnant or lactating women; (8) presence of magnetic resonance imaging (MRI) scan contraindications, including metal implants, pacemakers or stents, and claustrophobia. This study obtained written informed consent and was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University.
Assessment tool
All participants were required to provide general information and undergo psychological assessments, such as the Self-Rating Anxiety Scale (SAS) [24], the Self-Rating Depression Scale (SDS) [25] and the 24-item Hamilton Depression Rating Scale (HAMD) [26]. CT was quantified in this study using a 28-item Childhood Trauma Questionnaire (CTQ). This is a mature tool designed to quantify the psychological impact of trauma experienced before the age of 16 [27]. In our study, we employed the Chinese version of the CTQ, which yielded results indicating satisfactory internal consistency within the Chinese sample for both the total CTQ score (Cronbach’s α = 0.81) [28]. The CTQ includes five different subtypes of CT: emotional abuse, emotional neglect, sexual abuse, physical abuse, and physical neglect. The severity of CT can be quantified through the total score. Each subscale comprises five items, and participants are required to rate the extent to which each item applies to them on a scale of 1 = never true, 2 = rarely true, 3 = sometimes true, 4 = often true, 5 = almost always true. Scores on each subscale range from 5 to 25, with higher scores indicating more severe and prolonged CT [27]. To determine whether participants have experienced CT, we applied the following CTQ subscale thresholds: emotional abuse ≥ 13, emotional neglect ≥ 15, sexual abuse ≥ 8, physical abuse ≥ 10, and physical neglect ≥ 10. Scores exceeding any of these component scale thresholds are considered indicative of a history of CT. These thresholds have been validated and demonstrate good sensitivity and specificity to abuse or neglect [27].
MRI acquisition
MRI data were acquired at the Magnetic Resonance Center affiliated with the Second Xiangya Hospital of Central South University, utilizing a Philips 3.0-T scanner (Philips, Best, The Netherlands). Participants were instructed to recline within the scanner with their eyes closed. To minimize head movement, standard birdcage head coils were employed, along with foam pads placed on either side of the head, while cotton plugs were utilized to reduce noise interference. High-resolution T1-weighted anatomical images were captured for each participant using a three-dimensional rapid acquisition gradient echo sequence. Imaging of the entire brain was conducted in the sagittal plane, employing the following parameters: slice thickness = 1 mm, gap = 0 mm, repetition time = 7.6 ms, echo time = 3.7 ms, inversion time = 795 ms, field of view = 256 × 256 mm², flip angle = 8°, matrix size = 256 × 256, resolution = 1.0 × 1.0 × 1.0, slices = 180, scan time = 2′58’’.
Image processing
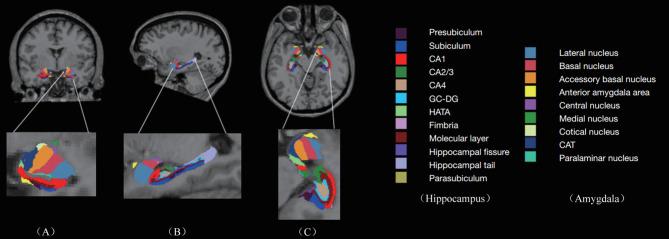
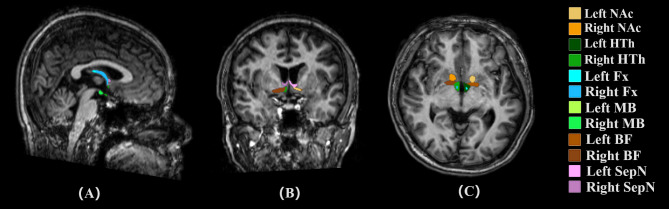
In our research, we leveraged FreeSurfer v7.2.0 to conduct precise subregional segmentation, targeting corticolimbic structures such as the hippocampus, brainstem, thalamus, hypothalamus, and amygdala. FreeSurfer provides a robust framework for processing structural MRI data, which includes critical steps such as skull stripping, B1 bias field correction, and gray-white matter segmentation. It also supports the reconstruction of cortical surface models, including the gray-white interface and pial surface, as well as the annotation of cortical and subcortical brain structures using stereotaxic atlases. Furthermore, FreeSurfer facilitates statistical analyses of morphometric variations across population cohorts through the nonlinear registration of individual cortical surfaces. We specifically used the hippocampus/amygdala module (https://surfer.nmr.mgh.harvard.edu/fswiki/HippocampalSubfieldsAndNucleiOfAmygdala) for the automated volumetric quantification of the hippocampus and amygdala from T1-weighted images. This module enables the simultaneous segmentation of the hippocampus and amygdala, avoiding any overlap or gaps between these structures. The segmentation process divides the left and right hippocampus into subregions (head, body, and tail) and partitions the amygdala into nine distinct nuclei, including lateral, basal, accessory basal, central, medial, cortical, and paralaminar nuclei, as well as the cortico-amygdaloid transition and anterior amygdala areas [29] (Fig. 1). This comprehensive approach ensures accurate characterization and differentiation of these important brain regions. Concurrently, we employ the deep learning tool mri_sclimbic_seg (https://surfer.nmr.mgh.harvard.edu/fswiki/ScLimbic) to autonomously delineate multiple subcortical limbic structures from T1-weighted images. This tool facilitates the automatic segmentation of several crucial subcortical limbic structures—including the nucleus accumbens(NAc), basal forebrain(BF), septal nuclei(SepN), hypothalamus without mammillary bodies(HTh), the mammillary bodies(MB), and fornix(Fx))—solely utilizing T1-weighted MRI data. Notably, tools for segmenting mammillary bodies, basal forebrain, septal nuclei, and fornix are currently scarce in published literature, thus underscoring the significance of our tool in addressing this gap [30] (Fig. 2).
Fig. 1.
Automatic Segmentation of Hippocampus and Amygala: The image shows the automatic segmentation of hippocampus(including presubiculum, subiculum, CA1, CA2/3, CA4, DC-DG, HATA, fimbria, Molecular layer, hippocampal fissure, hippocampal tail, parasubiculum) and amygdala(lateral nucleus, basal nucleus, accessory basal nucleus, anterior amygdala area, central nucleus, medial nucleus, cotical nucleus, CAT, paralaminar nucleus)—using T1-weighted MRI data
Fig. 2.
Automatic Segmentation of Subcortical Limbic Structures: The image shows the automatic segmentation of several crucial subcortical limbic structures—including the nucleus accumbens (NAc), basal forebrain (BF), septal nuclei (SepN), hypothalamus without mammillary bodies (HTh), mammillary bodies (MB), and fornix (Fx)—using T1-weighted MRI data
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (SPSS 26.0, IBM, Armonk, NY, USA). The continuous variables and categorical variables between the two groups were tested using independent two-sample t-tests and chi-square tests (χ2) respectively. The average estimated Total Intracranial Volume (eTIV) of all participants is calculated to derive the mean eTIV (meTIV). The volume of each brain region was then multiplied by meTIV/eTIV to correct for the effect of head size. The Benjamini-Hochberg method (BH) was used for false discovery rate (FDR) correction to adjust the p-values. Finally, using eTIV as a covariate, further correlation analyses were conducted between the CTQ scores and the volumes of various brain regions. The significance level for all tests was set at p < 0.05 for two-tailed analysis.
Results
Sample characteristics
As outlined in Table 1, our analysis revealed no statistically significant differences between the two participant groups across various demographic and psychological parameters. Specifically, there were no significant disparities observed in age (t = -0.075, p = 0.940), gender distribution (χ2 = 0.000, p = 1.000), educational attainment (t = -1.407, p = 0.166), as well as scores on the SAS (t = 1.430, p = 0.160) and the SDS (t = 1.014, p = 0.316). However, consistent with our hypotheses, significant distinctions were evident between the two experimental cohorts concerning the CTQ and its respective subscales, excluding sexual abuse (t = 3.234∼11.38, p < 0.01). Remarkably, emotional neglect emerged as the most prevalent CT experience within our sample, with 70.8% of participants reporting such exposure, while 62.5% of trauma-exposed individuals reported encountering at least two forms of CT.
Table 1.
Demographic and clinical variables of all subjects
| HP-CT group n = 24 means (SD) | HP-nCT group n = 24 means (SD) | t/χ2 | p-values | |
|---|---|---|---|---|
| Age (Years) | 21.50 (3.98) | 21.50 (3.69) | −0.075 | 0.940 |
| Gender (Male/Female) | 9/15 | 9/15 | 0.000 | 1.000 |
| Educational level (Years) | 14.00 (1.30) | 14.70 (1.92) | −1.407 | 0.166 |
| SDS score | 36.20 (6.06) | 34.50 (5.30) | 1.014 | 0.316 |
| SAS score | 34.00 (4.51) | 32.00 (4.78) | 1.430 | 0.160 |
| CTQ score | 0.11 (0.04) | 0.10 (0.03) | 0.835 | 0.408 |
| Emotional abuse | ||||
| Physical abuse | 9.21 (2.36) | 6.21 (1.22) | 5.539 | 0.000 |
| Sexual abuse | 7.83 (2.93) | 5.71 (1.33) | 3.324 | 0.002 |
| Emotional neglect | 5.46 (0.83) | 5.38 (0.58) | 0.403 | 0.689 |
| Physical neglect | 15.20 (3.28) | 7.38 (2.65) | 9.094 | 0.000 |
| Total | 10.20 (2.72) | 5.63 (0.93) | 7.821 | 0.000 |
| CTE, n (%) | 47.90 (6.08) | 30.20 (4.63) | 11.380 | 0.000 |
| Emotional abuse | ||||
| Physical abuse | 2.00 (8.33) | |||
| Sexual abuse | 8.00 (33.30) | |||
| Emotional neglect | 0 (0) | |||
| Physical neglect | 17.00 (70.80) | |||
| Multiply Exposures | 14.00 (58.30) | |||
| Single Exposure | 15.00 (62.50) |
HP-CT, Healthy participants with Childhood Trauma; HP-nCT, Healthy participants without Childhood Trauma; CTQ, Childhood Trauma Questionnaire; SAS, Self-rating Anxiety Scale; SD, Standard Deviation; SDS, Self-rating Depression Scale
Limbic system-related volume changes
We found that HP-CT group had smaller volumes in several subregions of the hippocampus, amygdala, and cortical limbic structures, including the Sub head (t = -2.804, p = 0.007) and body (t = -2.294, p = 0.026), CA1 head (t = -2.175, p = 0.035), ML head (t = -2.251, p = 0.029), GC-ML-DG body (t = -2.326, p = 0.024), CA4 body (t = -2.294, p = 0.026), fimbria (t = -2.106, p = 0.041), HATA (t = -2.313, p = 0.026), whole hippocampus head (t = -2.263, p = 0.028) and body (t = -2.433, p = 0.019), whole hippocampus (t = -2.704, p = 0.010), Ba (t = -2.059, p = 0.023), AB (t = -2.092, p = 0.042), CAT (t = -2.125, p = 0.042), PL (t = -2.177, p = 0.007), whole amygdala (t = -2.380, p = 0.022) of the left hemisphere; and hippocampal tail (t = -2.357, p = 0.023), PreSub body (t = -2.092, p = 0.042), and basal forebrain (t = -2.888, p = 0.006) of the right hemisphere. Notably, volumetric shifts within the amygdala were predominantly manifested in the left hemisphere. The discrepant areas failed to pass the BH correction. The detailed information for limbic system-related volumes of each group is summarized in Table 2.
Table 2.
Limbic system subfield volumes with significant differences among the two groups
| Region | Hemisphere | Subfields | Groups | Mean(SD) | t | p | Cohen’d |
|---|---|---|---|---|---|---|---|
| Hippocampus | Left | Sub head | HP-CT | 169.20 (17.68) | -2.804 | 0.007 | -0.81 |
| HP-nCT | 184.92 (21.01) | ||||||
| Sub body | HP-CT | 245.61 (21.18) | -2.294 | 0.026 | -0.66 | ||
| HP-nCT | 260.13 (22.65) | ||||||
| CA1 head | HP-CT | 490.89 (52.74) | -2.175 | 0.035 | -0.63 | ||
| HP-nCT | 524.59 (54.57) | ||||||
| ML head | HP-CT | 309.85 (33.03) | -2.251 | 0.029 | -0.65 | ||
| HP-nCT | 331.71 (34.22) | ||||||
| GC-ML-DG body | HP-CT | 140.95 (13.25) | -2.326 | 0.024 | -0.67 | ||
| HP-nCT | 150.32 (14.64) | ||||||
| CA4 body | HP-CT | 122.23(11.42) | -2.294 | 0.026 | -0.66 | ||
| HP-nCT | 130.67 (13.95) | ||||||
| Fimbria | HP-CT | 72.95 (11.89) | -2.106 | 0.041 | -0.61 | ||
| HP-nCT | 81.06 (14.65) | ||||||
| HATA | HP-CT | 56.74 (9.53) | -2.313 | 0.026 | -0.67 | ||
| HP-nCT | 62.18 (6.50) | ||||||
| Whole hippocampus head | HP-CT | 1612.80 (171.74) | -2.263 | 0.028 | -0.65 | ||
| HP-nCT | 1724.81 (171.20) | ||||||
| Whole hippocampus body | HP-CT | 1204.75 (91.19) | -2.433 | 0.019 | -0.70 | ||
| HP-nCT | 1269.38 (92.82) | ||||||
| Whole hippocampus | HP-CT | 3442.09 (304.01) | -2.704 | 0.010 | -0.78 | ||
| HP-nCT | 3666.24 (269.50) | ||||||
| HP-nCT | 73.01 (12.32) | ||||||
| Right | Hippocampal tail | HP-nCT | 589.77 (57.32) | -2.357 | 0.023 | -0.68 | |
| HP-nCT | 632.28 (67.23) | ||||||
| Presubiculum body | HP-nCT | 144.90 (22.27) | -2.092 | 0.042 | -0.60 | ||
| HP-nCT | 161.01 (30.48) | ||||||
| Amygdala | Left | Ba | HP-CT | 418.17 (39.93) | -2.059 | 0.014 | -0.74 |
| HP-nCT | 447.43 (39.09) | ||||||
| AB | HP-CT | 246.98 (23.67) | -2.053 | 0.020 | -0.69 | ||
| HP-nCT | 262.44 (20.82) | ||||||
| CAT | HP-CT | 176.78 (18.50) | -2.125 | 0.042 | -0.60 | ||
| HP-nCT | 187.27 (16.28) | ||||||
| PL | HP-CT | 48.89(5.05) | -2.177 | 0.007 | -0.82 | ||
| HP-nCT | 52.77 (4.42) | ||||||
| Whole amygdala | HP-CT | 1660.37 (129.25) | -2.380 | 0.022 | -0.69 | ||
| HP-nCT | 1754.64 (144.71) | ||||||
| Cortical limbic structures | Right | BF | HP-CT | 276.86 (46.67) | -2.888 | 0.006 | -0.83 |
| HP-nCT | 309.36 (29.36) |
SD, standard deviation; HP-CT, healthy participants with childhood trauma; HP-nCT, healthy participants without childhood trauma; Sub, subiculum; CA, cornu ammonis; ML, molecular layer; GC-ML-DG, granule cell layer of the dentate gyrus; HATA, hippocampus-amygdala transition area; Ba, basal nucleus; AB, accessory basal nucleus; CAT, cortico-amygdaloid transition area; PL, paralaminar nucleus; BF, basal forebrain
Correlations
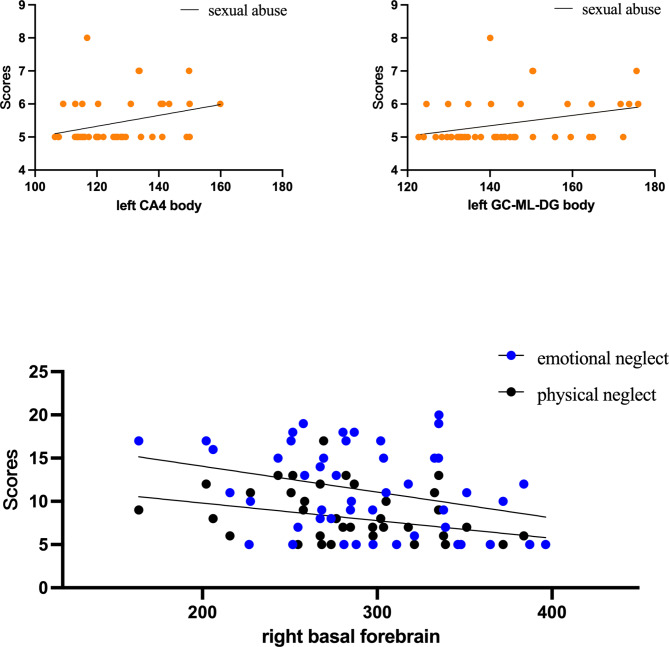
As shown in Fig. 3, the volume changes of the left CA4 body (rs = 0.310, p = 0.032) and left GC-ML-DG body (rs = 0.331, p = 0.022) are associated with sexual abuse. Changes in the volume of the right basal forebrain are linked to emotional neglect (rs = -0.311, p = 0.031) and physical neglect (rs = -0.341, p = 0.018). No volume changes were found in other subregions to be correlated with scores of CTQ.
Fig. 3.
Correlation between volume changes in each subregion and CTQ scores
Discussion
The correlation between traumatic events and selective structural deficiencies in the hippocampal subfields has been widely explored; however, there exist discrepancies among various reports within the field, [31, 32]. In our study, we also observed hippocampal volume reduction consistent with prior research; yet, within neuroimaging studies, numerous psychiatric disorders manifest alterations in hippocampal volume, suggesting the pivotal role the hippocampus plays as a part of neural circuits across various domains [33, 34]. Moreover, alterations in hippocampal morphology among CT survivors are not solely regulated by the presence or absence of lifelong emotional disorders; hence, the correlation between CT and hippocampal volume may hold independent significance from clinical diagnosis [35]. Previous literature indicates that adolescents exposed to trauma exhibit smaller volumes in the left presubiculum and subiculum [36], a region known for its crucial role in memory formation and retrieval via modulation of neural circuits [37]. One study suggests increased rightward lateralization of the hippocampal tail in early trauma [38]. The CA1 subfield plays a crucial role in PTSD because it establishes direct reciprocal connections with the medial prefrontal cortex (mPFC), creating a functional loop between these regions. This loop facilitates communication between cortical and subcortical regions during the encoding and retrieval of episodic-like memories [39]. Observations from prior trauma-related studies highlight volume changes in other classical hippocampal subfields, notably the strongest association between trauma and volume observed in the left CA2-CA3 and CA4-DG regions, independent of psychiatric diagnoses [36]. The correlation between CA3 and CT is influenced by age [32]. Some neurobiological models suggest that hippocampal volume reduction may stem from early exposure to elevated levels of stress-related corticotropin-releasing hormone (CRH), leading to hippocampal neuron degeneration [18, 40], a hypothesis supported by certain clinical studies [41]. Limited results have reported the role of the HATA subfield in trauma. Typically, the amygdala and hippocampus communicate bidirectionally, with the basolateral amygdala anatomically poised to regulate hippocampal function and synaptic plasticity through interaction with the hippocampus [42]. HATA itself is closely linked with the amygdala within the hippocampal-amygdalar network, believed to play a pivotal role in fear regulation and contextual learning. A cross-sectional study suggests an interaction between early traumatic events and the rs1360780 polymorphism of the FKBP5 gene within the hypothalamic-pituitary-adrenal (HPA) axis in HATA [43]. Pathways associated with FKBP5 may also be implicated in the pathogenesis of PTSD. A neuroimaging genetics study suggests a strong link between volume changes in Fimbria and trauma-related genes [44]. In summary, these findings underscore the significance of the subiculum, presubiculum, hippocampal tail, the CA1, the CA4-DG subfield, and HATA in CT.
As a closely associated structure in CT, research findings on the volume changes of the amygdala remain inconsistent. The volume alterations in the amygdala may correlate with the timing and duration of trauma exposure. Smaller amygdala volumes have been observed in individuals with CT and PTSD [9], while larger volumes are reported in adult [10]. Short-duration stress events show a correlation with amygdala size across the sample, whereas longer events do not, suggesting rapid and reversible changes independent of depressive states [45]. Our study observed reduced volumes in the left Ba, AB, CAT, and PL in trauma-exposed healthy individuals. Among these, Ba, PL, and AB belong to the basolateral amygdala (BLA), primarily responsible for receiving sensory information, including inputs from the hippocampus and primary auditory cortex, and is involved in emotional arousal [46]. Previous research has identified associations between trauma and the basolateral and central-medial nuclei of the amygdala [47], as well as the Ba, AB, and cortical subnuclei (Ce) [48], amygdala medial nuclei (Me), and cortical nucleus (Co) [49], often exhibiting right-sided biases, confounded by factors such as psychiatric disorders. Contrary to prior studies, our results demonstrate a different lateralization pattern. Opposing patterns of lateralization might be better understood from a neurodevelopmental perspective. The innervation of neurotransmitter systems in the brain is lateralized, and this lateralization is exacerbated by early-life stress [47]. Inconsistent findings regarding amygdala volume may also be influenced by the timing of trauma and the presence of psychiatric disorders [10]. Therefore, lateralization could potentially be disrupted by factors such as the timing of trauma. To fully understand these lateralization patterns, future longitudinal studies will be essential to further validate these observations. The flow of information within the amygdala is modulated by GABAergic neurons, which are disrupted after trauma, leading to excessive excitation and the development of anxiety or other emotional disorders. The rich expression of neuroregulatory receptors within the BLA also renders it a target for psychiatric disease treatment [50, 51]. CAT has been less frequently mentioned in previous trauma-related studies, though prior research suggests different connectivity patterns between CAT and the orbitofrontal cortex (OFC) compared to other amygdala subregions [52]. Overall, the BLA and CAT of the amygdala likely play distinct roles in trauma through different circuits.
As part of the limbic system, we haven’t previously observed correlations between the thalamus and CT. Studies have shown that as the severity of trauma increases, the overall volume of the thalamus and its subregions decreases [53], a conclusion also supported by large-scale cohort studies [11]. In PTSD patients, significant reductions in gray matter volume have been observed in the left thalamus and its subregions, including the anterior thalamic nucleus, mediodorsal thalamus, ventrolateral-dorsal (VLD), ventrolateral-anterior, and ventrolateral-ventral (VLV) regions [54]. The thalamus itself has been implicated in fear response pathways along with the basolateral nucleus of the amygdala, a pathway closely associated with trauma [55], and may also participate in the pathway from the superior colliculus to the amygdala (superior colliculus-pulvinar-amygdala connection) involved in trauma onset [56]. The central medial nucleus on the right side connects with structures associated with the limbic system and functions related to stress/anxiety [57]. In mouse models, it has been demonstrated that the central medial nucleus participates in anxiety-like behaviors through the CM-mPFC pathway [58]. Although our study did not identify distinct volumetric disparities in the thalamic subregions between the two groups, existing research has indicated potential differences in thalamic functional connectivity among major depressive disorder (MDD) patients with a history of trauma [59]. This suggests the need for further exploration in healthy individuals to gain a more nuanced understanding of the role of thalamic functional connectivity in relation to trauma and its impact on brain function. Previously, a reduction in gray matter volume in the right middle cingulate gyrus was observed in healthy subjects [22]. However, we did not observe significant volume changes in the cingulate cortex between groups with and without childhood trauma. Caution is needed when interpreting why this change was not detected in individuals without psychiatric disorders. It is possible that cingulate volume changes may only become evident in larger samples or longitudinal studies.
Our study has unveiled notable lateralized manifestations, warranting in-depth exploration. In our study, we observed noticeable unilateral volume differences in specific brain regions, including the right basal forebrain, right hippocampal tail, right PreSub body, left Sub head and body, left CA1 head, left ML head, left GC-ML-DG body, left fimbria, left HATA, left Ba, left AB, left CAT. Prior research has indicated a potential correlation between larger volumes of the left amygdala and increased exposure to trauma [60]. A longitudinal study has demonstrated differential treatment responses between the left and right amygdalae, lending support to this assertion [61]. Females with CT history and veterans commonly exhibit diminished volume in the left hippocampus [62]. Emotional trauma is also more likely to induce alterations in the volume of the left CA3 subregion of the hippocampus [32]. Furthermore, studies about psychiatric disorders suggest that the relationship between adverse childhood experience scores and amygdala measurements predominantly manifests on the right side [47]. Functional and structural disparities exist between the left and right hemispheres. While traditional views regard lateralization as a static trait, emerging research suggests that the brain necessitates continuous adjustments in its activity and coordinated patterns of interregional activity to accommodate evolving environmental demands and achieve complex cognitive function [63]. From a neurodevelopmental perspective, child abuse is associated with delayed myelination of the corpus callosum and reduced corpus callosum volume is linked to diminished interhemispheric communication, thereby fostering hemisphere-specific development to some extent [64]. Recent investigations have uncovered asymmetrical differences in electroencephalographic waveforms among trauma-exposed children within healthy cohorts [65]. Currently, no consensus exists regarding the relationship between volumetric changes and functional connectivity, necessitating further elucidation through future research endeavors. Moreover, a unified cognitive perspective on cerebral lateralization remains elusive, with the dominant hemisphere exhibiting plasticity and dynamic variability. Although lateralization is observed, we cannot assume that CT may affect cognitive function by shaping the lateralized structural organization of the brain.
Different types of CT exhibit distinct associations with volumetric changes across the limbic system. Specifically, alterations in the CA4 body and GC-ML-DG body regions of the hippocampus were positively correlated with scores indicative of sexual abuse. Notably, volume changes in the right basal forebrain showed a negative correlation with scores related to emotional neglect and somatic neglect. The diverse impacts of various forms of abuse on neurodevelopment are apparent, with experiences of physical aggression and threats of violence potentially engender differing degrees of neurodevelopmental consequences. Consequently, discernible neurodevelopmental disparities may exist among distinct traumatic experiences [17]. Future investigations should delve deeper into the nuanced effects of different types of CT on limbic system volume to enhance our comprehension of these underlying mechanisms. Importantly, given the limitations imposed by our small sample size, caution is warranted when interpreting these correlation results. Thus, careful consideration of the influence of sample size on the findings is essential in their interpretation.
In explicating our findings, it is crucial to acknowledge certain limitations. The study’s small sample size restricts the comparison of various CT types on brain structural changes. Larger samples could offer more reliable results. The methodology, relying on participant recall and questionnaire completion, may introduce memory and information biases, potentially compromising accuracy. The cross-sectional design hampers causal inference, though it provides initial insights for further research. Moreover, our findings did not remain significant after FDR correction, but we reported effect sizes to ensure a more accurate interpretation of results as suggested by previous studies [66, 67]. Although the use of FreeSurfer to obtain volumetric data requires less time compared to manual delineation, there are still certain concerns regarding its accuracy [68–70]. Given the size and anterior-posterior transition of hippocampal subfields and amygdalar subnuclei, future studies may need to employ submillimeter voxel sizes [71]. Despite these constraints, the study suggests an association between CT and volumetric changes in limbic subregions, possibly exhibiting lateralization. Furthermore, correlations between CTQ scores and specific structural changes were noted. Comparative analyses with healthy and psychiatric cohorts indicate that limbic system alterations may signal vulnerability to psychiatric conditions following CT.
Conclusion
In summary, CT affects multiple structures of the limbic system, including the hippocampus, and amygdala. This also suggests that region-specific changes within the limbic system could serve as clinical biomarkers to support cross-diagnosis of psychiatric disorders. And there is a certain correlation between different types of CT and volume changes, which requires further research in the future. The association between lateralized manifestations and CT still requires further investigation to understand the underlying mechanisms driving these findings.
Acknowledgements
The authors would like to thank all participants who took part in this study, and the experts at the Magnetic Resonance Center of The Second Xiangya Hospital for providing scan time and technical assistance.
Abbreviations
- CT
Childhood trauma
- HP-CT
Healthy participants with CT
- HP-nCT
Healthy participants without CT
- Sub
Subiculum
- CA
Cornu ammonis
- ML
Molecular layer
- GC-ML-DG
Granule cell layer of the dentate gyru
- HATA
Hippocampus-amygdala transition area
- Ba
Basal nucleus
- AB
Accessory basal nucleus
- CAT
Cortico-amygdaloid transition area
- PL
Paralaminar nucleus
- PreSub
Presubiculum
- PTSD
Post-traumatic stress disorder
- CRP
C-reaction protein
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- SCID
Structured clinical interview for DSM-IV
- MRI
Magnetic resonance imaging
- SAS
Self-Rating Anxiety Scale
- SDS
Self-Rating Depression Scale
- HAMD
Hamilton Depression Rating Scale
- CTQ
Childhood Trauma Questionnaire
- NAc
Nucleus accumbens
- BF
Basal forebrain
- SepN
Septal nuclei
- HTh
Hypothalamus without mammillary bodies
- MB
Mammillary bodies
- Fx
Fornix
- eTIV
Estimated Total Intracranial Volume
- meTIV
Mean estimated Total Intracranial Volume
- mPFC
Medial prefrontal cortex
- HPA
Hypothalamic-pituitary-adrenal
- BLA
Basolateral amygdala
- Ce
Cortical subnuclei
- Me
Amygdala medial nuclei
- Co
Cortical nucleus
- OFC
Orbitofrontal cortex
- VLD
Ventrolateral-dorsal
- VLV
Ventrolateral-ventral
- MDD
Major depressive disorder
Author contributions
Author Yuwei Xu wrote the first draft of the manuscript. Authors Shaojia Lu and Lingjiang Li designed the study, recruited the sample, and finished the clinical assessments. Authors Dong Cui, Manli Huang, and Shaohua Hu conducted the statistical analyses. Authors Lingjiang Li and Lei Zhang also designed the study and had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to and have approved the final manuscript.
Funding
This work was supported by the STI2030-Major Project (2021ZD0200600 to Shaojia Lu), National Natural Science Foundation of China (82071521 to Shaojia Lu), and Natural Science Foundation of Zhejiang Province (LY19H090017 to Shaojia Lu).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethic committee of the Second Xiangya Hospital, Central South University. All subjects provided written informed consent prior to participation. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaojia Lu and Yuwei Xu contributed equally to this work.
Contributor Information
Shaojia Lu, Email: 1314004@zju.edu.cn.
Lingjiang Li, Email: llj2920@163.com.
Lei Zhang, Email: 33382746@qq.com.
References
- 1.González-Acosta CA, Rojas-Cerón CA, Buriticá E. Functional alterations and cerebral variations in humans exposed to early life stress. Front Public Health. 2020;8:536188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Psychiatry null. Responding to childhood trauma. Lancet Psychiatry. 2022;9:759. [DOI] [PubMed] [Google Scholar]
- 3.Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, et al. Childhood adversity impacts on brain subcortical structures relevant to depression. J Psychiatr Res. 2017;86:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aust S, Stasch J, Jentschke S, Alkan Härtwig E, Koelsch S, Heuser I, et al. Differential effects of early life stress on hippocampus and amygdala volume as a function of emotional abilities. Hippocampus. 2014;24:1094–101. [DOI] [PubMed] [Google Scholar]
- 5.Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. [DOI] [PubMed] [Google Scholar]
- 7.Nogovitsyn N, Addington J, Souza R, Placsko TJ, Stowkowy J, Wang J, et al. Childhood trauma and amygdala nuclei volumes in youth at risk for mental illness. Psychol Med. 2022;52:1192–9. [DOI] [PubMed] [Google Scholar]
- 8.Rl A, Rkr HLTIMW et al. S, N K,. Amygdala subnucleus volumes in psychosis high-risk state and first-episode psychosis. Schizophrenia research. 2020 [cited 2024 Oct 14];215. https://pubmed.ncbi.nlm.nih.gov/31744752/ [DOI] [PubMed]
- 9.Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SARB. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res. 2015;233:436–42. [DOI] [PubMed] [Google Scholar]
- 10.Siehl S, Sicorello M, Herzog J, Nees F, Kleindienst N, Bohus M, et al. Neurostructural associations with traumatic experiences during child- and adulthood. Transl Psychiatry. 2022;12:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madden RA, Atkinson K, Shen X, Green C, Hillary RF, Hawkins E, et al. Structural brain correlates of childhood trauma with replication across two large, independent community-based samples. Eur Psychiatry. 2023;66:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry. 1999;45:1271–84. [DOI] [PubMed] [Google Scholar]
- 13.Rinne-Albers MA, Pannekoek JN, van Hoof M-J, van Lang ND, Lamers-Winkelman F, Rombouts SA, et al. Anterior cingulate cortex grey matter volume abnormalities in adolescents with PTSD after childhood sexual abuse. Eur Neuropsychopharmacol. 2017;27:1163–71. [DOI] [PubMed] [Google Scholar]
- 14.Ross MC, Heilicher M, Cisler JM. Functional imaging correlates of childhood trauma: a qualitative review of past research and emerging trends. Pharmacol Biochem Behav. 2021;211:173297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Guan X, Li H, Zhang M, Xu X. The effect of early life stress on memory is mediated by anterior hippocampal network. Neuroscience. 2020;451:137–48. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij SJH, Smith RD, Stenson AF, Ely TD, Yang X, Tottenham N, et al. Increased activation of the fear neurocircuitry in children exposed to violence. Depress Anxiety. 2020;37:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaney SW, Cortes Hidalgo AP, White T, Haneuse S, Ressler KJ, Tiemeier H, et al. Are all threats equal? Associations of childhood exposure to physical attack versus threatened violence with preadolescent brain structure. Dev Cogn Neurosci. 2021;52:101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al YC, Tz AMF. B. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Frontiers in cellular neuroscience. 2012 [cited 2024 Feb 26];6. https://pubmed.ncbi.nlm.nih.gov/22514519/ [DOI] [PMC free article] [PubMed]
- 19.Kl B, M E-A RB, Tz YC. B. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences of the United States of America. 2001 [cited 2024 Oct 14];98. https://pubmed.ncbi.nlm.nih.gov/11447269/ [DOI] [PMC free article] [PubMed]
- 20.Hakamata Y, Suzuki Y, Kobashikawa H, Hori H. Neurobiology of early life adversity: a systematic review of meta-analyses towards an integrative account of its neurobiological trajectories to mental disorders. Front Neuroendocrinol. 2022;65:100994. [DOI] [PubMed] [Google Scholar]
- 21.Panzer A. The neuroendocrinological sequelae of stress during brain development: the impact of child abuse and neglect. Afr J Psychiatry (Johannesbg). 2008;11:29–34. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, Gao W, Wei Z, Wu W, Liao M, Ding Y, et al. Reduced cingulate gyrus volume associated with enhanced cortisol awakening response in young healthy adults reporting childhood trauma. PLoS ONE. 2013;8:e69350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Förster K, Danzer L, Redlich R, Opel N, Grotegerd D, Leehr EJ, et al. Social support and hippocampal volume are negatively associated in adults with previous experience of childhood maltreatment. J Psychiatry Neurosci. 2021;46:E328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–9. [DOI] [PubMed] [Google Scholar]
- 25.Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13:508–15. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–8. [DOI] [PubMed] [Google Scholar]
- 28.Jiang W-J, Zhong B-L, Liu L-Z, Zhou Y-J, Hu X-H, Li Y. Reliability and validity of the Chinese version of the Childhood Trauma Questionnaire-Short Form for inpatients with schizophrenia. PLoS ONE. 2018;13:e0208779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. NeuroImage. 2017;155:370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greve DN, Billot B, Cordero D, Hoopes A, Hoffmann M, Dalca AV, et al. A deep learning toolbox for automatic segmentation of subcortical limbic structures from MRI images. NeuroImage. 2021;244:118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrova LI, Dean SL, Schlumpf YR, Vissia EM, Nijenhuis ERS, Chatzi V, et al. A neurostructural biomarker of dissociative amnesia: a hippocampal study in dissociative identity disorder. Psychol Med. 2023;53:805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhi GS, Das P, Outhred T, Irwin L, Gessler D, Bwabi Z, et al. The effects of childhood trauma on adolescent hippocampal subfields. Aust N Z J Psychiatry. 2019;53:447–57. [DOI] [PubMed] [Google Scholar]
- 33.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a Multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder Consortia. Biol Psychiatry. 2018;83:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips RD, De Bellis MD, Brumback T, Clausen AN, Clarke-Rubright EK, Haswell CC, et al. Volumetric trajectories of hippocampal subfields and amygdala nuclei influenced by adolescent alcohol use and lifetime trauma. Transl Psychiatry. 2021;11:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opel N, Redlich R, Zwanzger P, Grotegerd D, Arolt V, Heindel W, et al. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology. 2014;39:2723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109:E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ds R, T K, Sk TO, C O et al. S, Y O,. Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell. 2017 [cited 2024 Feb 26];170. https://pubmed.ncbi.nlm.nih.gov/28823555/ [DOI] [PMC free article] [PubMed]
- 38.Lee J, Song Y, Won E, Bang M, Lee S-H. Higher Rightward Laterality of the Hippocampal Tail and Its Association with early trauma in panic disorder. Clin Psychopharmacol Neurosci. 2020;18:311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–79. [DOI] [PubMed] [Google Scholar]
- 41.Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EMM, et al. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology. 2011;214:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakao K, Matsuyama K, Matsuki N, Ikegaya Y. Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:14270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikolas P, Tozzi L, Doolin K, Farrell C, O’Keane V, Frodl T. Effects of early life adversity and FKBP5 genotype on hippocampal subfields volume in major depression. J Affect Disord. 2019;252:152–9. [DOI] [PubMed] [Google Scholar]
- 44.Morey RA, Garrett ME, Stevens JS, Clarke EK, Haswell CC, van Rooij SJH, et al. Genetic predictors of hippocampal subfield volume in PTSD cases and trauma-exposed controls. Eur J Psychotraumatol. 2020;11:1785994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sublette ME, Galfalvy HC, Oquendo MA, Bart CP, Schneck N, Arango V, et al. Relationship of recent stress to amygdala volume in depressed and healthy adults. J Affect Disord. 2016;203:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang W, Kochubey O, Kintscher M, Schneggenburger R. A VTA to basal amygdala dopamine projection contributes to signal salient somatosensory events during fear learning. J Neurosci. 2020;40:3969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshri A, Gray JC, Owens MM, Liu S, Duprey EB, Sweet LH, et al. Adverse childhood experiences and Amygdalar reduction: high-resolution segmentation reveals associations with Subnuclei and Psychiatric outcomes. Child Maltreat. 2019;24:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aghamohammadi-Sereshki A, Coupland NJ, Silverstone PH, Huang Y, Hegadoren KM, Carter R, et al. Effects of childhood adversity on the volumes of the amygdala subnuclei and hippocampal subfields in individuals with major depressive disorder. J Psychiatry Neurosci. 2021;46:E186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Lu L, Bu X, Li H, Tang S, Gao Y, et al. Alterations in hippocampal subfield and amygdala subregion volumes in posttraumatic subjects with and without posttraumatic stress disorder. Hum Brain Mapp. 2021;42:2147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prager EM, Bergstrom HC, Wynn GH, Braga MFM. The basolateral amygdala γ-aminobutyric acidergic system in health and disease. J Neurosci Res. 2016;94:548–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asede D, Doddapaneni D, Bolton MM. Amygdala intercalated cells: gate keepers and conveyors of Internal State to the circuits of emotion. J Neurosci. 2022;42:9098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma M, Jm S. Differential spatial patterns of structural connectivity of amygdala nuclei with orbitofrontal cortex. Human brain mapping. 2021 [cited 2024 Feb 26];42. https://pubmed.ncbi.nlm.nih.gov/33270320/ [DOI] [PMC free article] [PubMed]
- 53.Xie H, Huffman N, Shih C-H, Cotton AS, Buehler M, Brickman KR, et al. Adverse childhood experiences associate with early post-trauma thalamus and thalamic nuclei volumes and PTSD development in adulthood. Psychiatry Res Neuroimaging. 2022;319:111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang B, Jia Y, Zheng W, Wang L, Qi Q, Qin W, et al. Structural changes in the thalamus and its subregions in regulating different symptoms of posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2023;335:111706. [DOI] [PubMed] [Google Scholar]
- 55.Hájos N. Interneuron types and their circuits in the Basolateral Amygdala. Front Neural Circuits. 2021;15:687257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshii T. The role of the Thalamus in post-traumatic stress disorder. Int J Mol Sci. 2021;22:1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J-N, Wu X-M, Zhao L-J, Sun H-X, Hong J, Wu F-L, et al. Central medial thalamic nucleus dynamically participates in acute itch sensation and chronic itch-induced anxiety-like behavior in male mice. Nat Commun. 2023;14:2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu T, Zou Y, Nie H, Li Y, Chen J, Du Y, et al. The role of the thalamic subregions in major depressive disorder with childhood maltreatment: evidences from dynamic and static functional connectivity. J Affect Disord. 2023;347:237–48. [DOI] [PubMed] [Google Scholar]
- 60.Picci G, Taylor BK, Killanin AD, Eastman JA, Frenzel MR, Wang Y-P, et al. Left amygdala structure mediates longitudinal associations between exposure to threat and long-term psychiatric symptomatology in youth. Hum Brain Mapp. 2022;43:4091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joss D, Khan A, Lazar SW, Teicher MH. A pilot study on amygdala volumetric changes among young adults with childhood maltreatment histories after a mindfulness intervention. Behav Brain Res. 2021;399:113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–9. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Kong X, Vatansever D, Liu Z, Zhang K, Sahakian BJ, et al. Dynamic changes in brain lateralization correlate with human cognitive performance. PLoS Biol. 2022;20:e3001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10:150–72. [DOI] [PubMed] [Google Scholar]
- 65.Im S, Fitzpatrick S, Hien DA, Lopez-Castro T, Pawlak A, Melara RD. Frontal alpha asymmetry in children with trauma exposure. Clin EEG Neurosci. 2022;53:418–25. [DOI] [PubMed] [Google Scholar]
- 66.Otte WM, Vinkers CH, Habets PC, van IJzendoorn DGP, Tijdink JK. Analysis of 567,758 randomized controlled trials published over 30 years reveals trends in phrases used to discuss results that do not reach statistical significance. PLoS Biol. 2022;20:e3001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nead KT, Wehner MR, Mitra N. The Use of ‘Trend’ statements to describe statistically nonsignificant results in the Oncology Literature. JAMA Oncol. 2018;4:1778–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kahhale I, Buser NJ, Madan CR, Hanson JL. Quantifying numerical and spatial reliability of hippocampal and amygdala subdivisions in FreeSurfer. Brain Inf. 2023;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander B, Georgiou-Karistianis N, Beare R, Ahveninen LM, Lorenzetti V, Stout JC, et al. Accuracy of automated amygdala MRI segmentation approaches in Huntington’s disease in the IMAGE-HD cohort. Hum Brain Mapp. 2020;41:1875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eb RJ, Ja TBC, Pl T, Kl A et al. P,. Amygdala volume and social anxiety symptom severity: Does segmentation technique matter? Psychiatry research Neuroimaging. 2020 [cited 2024 Oct 14];295. https://pubmed.ncbi.nlm.nih.gov/31760338/ [DOI] [PMC free article] [PubMed]
- 71.Lem W, Am GC, R de F D, la Sg R et al. M,. Hippocampal subfield volumetry from structural isotropic 1 mm3 MRI scans: A note of caution. Human brain mapping. 2021 [cited 2024 Oct 14];42. https://pubmed.ncbi.nlm.nih.gov/33058385/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request.