Abstract
Purified rat renal brush-border membrane vesicles possess a heat-labile enzyme activity which hydrolyses NAD+. A reciprocal relationship exists between the disappearance of NAD+ and the appearance of adenosine; 2 mol of Pi are liberated from each mol of NAD+ incubated with brush-border membrane vesicles. Freezing and thawing brush-border membrane vesicles does not enhance the initial rate of NAD+ hydrolysis. Preincubation of brush-border membrane vesicles with NAD+ results in inhibition of Na+-dependent Pi-transport activity, whereas Na+-dependent glucose transport is not affected. EDTA, which prevents the release of Pi from NAD+ and which itself has no direct effect on brush-border membrane Pi transport, reverses the NAD+ inhibition of Na+-dependent Pi transport. These results suggest that it is the Pi liberated from NAD+ and not NAD+ itself that inhibits Na+-dependent Pi transport.
Full text
PDF
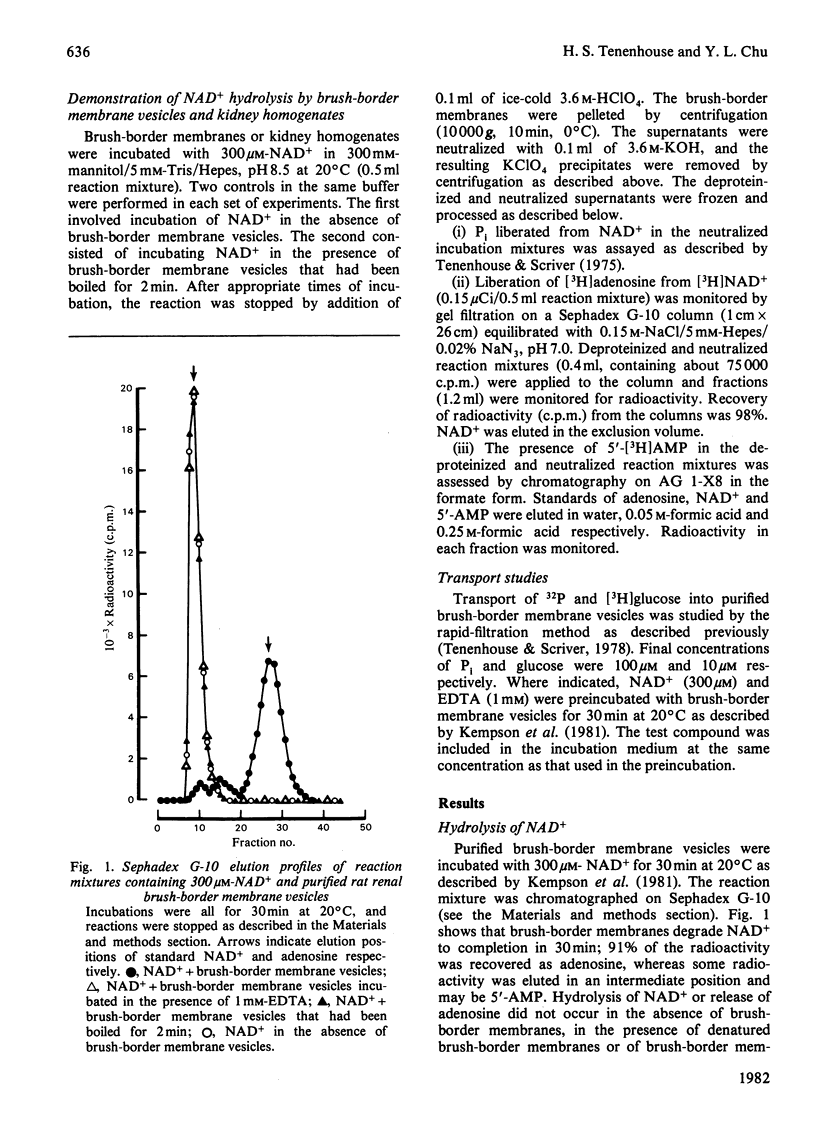
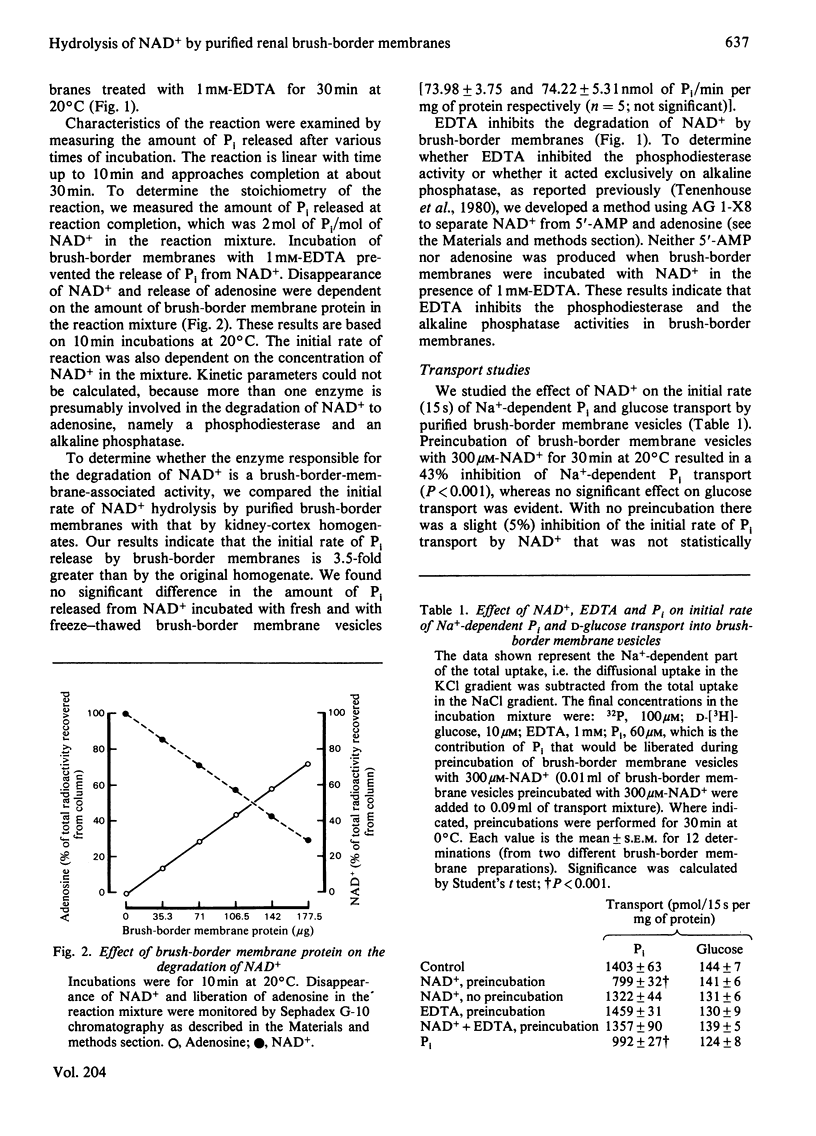

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berndt T. J., Knox F. G., Kempson S. A., Dousa T. P. Nicotinamide adenine dinucleotide and renal response to parathyroid hormone. Endocrinology. 1981 May;108(5):2005–2007. doi: 10.1210/endo-108-5-2005. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis V. W., Stead W. W., Myers J. L. Renal handling of phosphate and calcium. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- Filburn C. R., Sacktor B. Cyclic nucleotide phosphodiesterases of rabbit renal cortex. Characterization of brush border membrane activities. Arch Biochem Biophys. 1976 May;174(1):249–261. doi: 10.1016/0003-9861(76)90344-1. [DOI] [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson S. A., Colon-Otero G., Ou S. Y., Turner S. T., Dousa T. P. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest. 1981 May;67(5):1347–1360. doi: 10.1172/JCI110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox F. G., Schneider E. G., Willis L. R., Strandhoy J. W., Ott C. E. Editorial: Site and control of phosphate reabsorption by the kidney. Kidney Int. 1973 Jun;3(6):347–353. doi: 10.1038/ki.1973.56. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. Orthophosphate transport in the erythrocyte of normal subjects and of patients with X-linked hypophosphatemia. J Clin Invest. 1975 Mar;55(3):644–654. doi: 10.1172/JCI107972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. The defect in transcellular transport of phosphate in the nephron is located in brush-border membranes in X-linked hypophosphatemia (Hyp mouse model). Can J Biochem. 1978 Jun;56(6):640–646. doi: 10.1139/o78-096. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R., Vizel E. J. Alkaline phosphatase activity does not mediate phosphate transport in the renal-cortical brush-border membrane. Biochem J. 1980 Aug 15;190(2):473–476. doi: 10.1042/bj1900473. [DOI] [PMC free article] [PubMed] [Google Scholar]


