Abstract
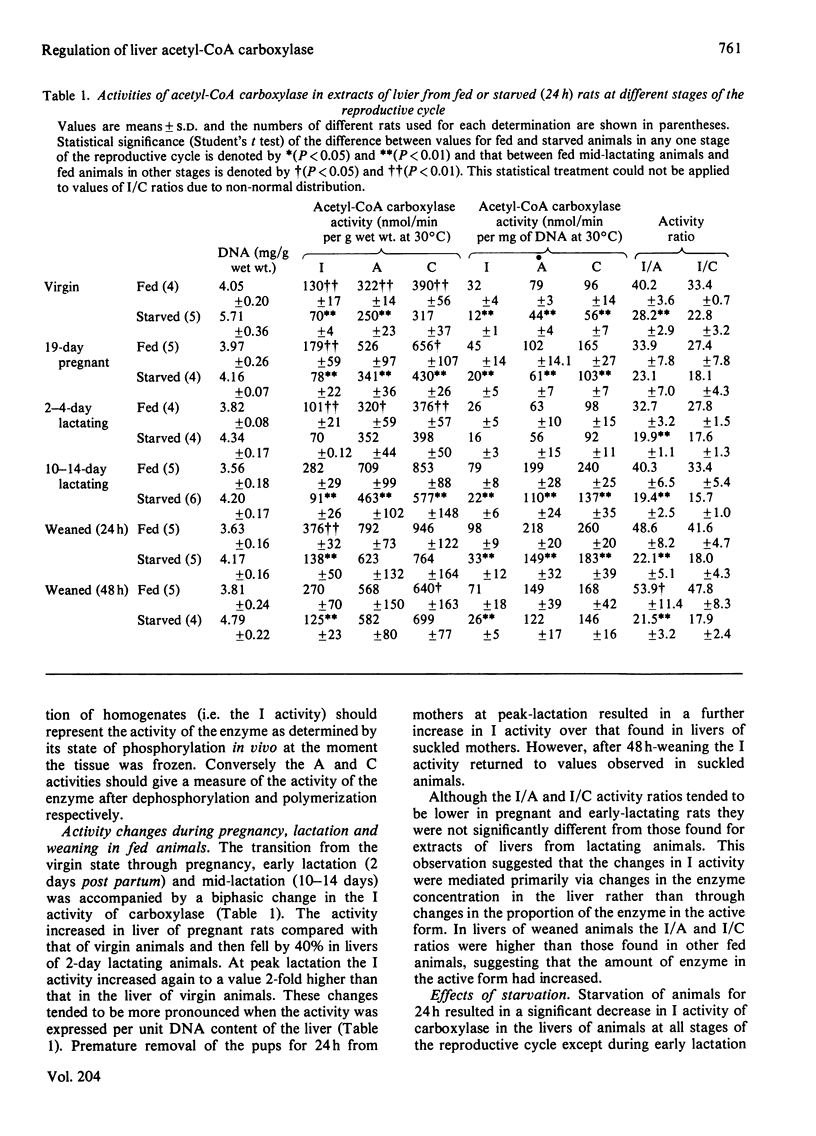
1. The activity of acetyl-CoA carboxylase (EC 6.4.1.2) in extracts of freeze-clamped liver samples from fed or 24 h-starved virgin, pregnant, lactating and weaned rats was measured (i) immediately after preparation of extracts (`I activity'), (ii) after incubation of extracts with partially purified preparations of either rabbit muscle protein phosphatase 1 [Antoniw, Nimmo, Yeaman & Cohen (1977) Biochem. J. 162, 423–433] or rabbit liver phosphatase [Brandt, Capulong & Lee (1975) J. Biol. Chem. 250, 8038–8044] (`A activity') and (iii) after incubation with 20mm-potassium citrate before or after incubation with phosphatases (`C activity'). 2. Incubation of liver extracts at 30°C without any additions resulted in activation of acetyl-CoA carboxylase that was shown to be due to dephosphorylation of the enzyme by endogenous protein phosphatase activity. This latter activity was not stimulated by Ca2+ and/or Mg2+ but was stimulated by 1 mm-Mn2+. Incubation of extracts with either of the partially purified phosphatases (0.2–0.5 unit) resulted in faster dephosphorylation and activation. The activity achieved after incubation with either of the exogenously added phosphatases was similar. 3. The A and C activities increased during late pregnancy, were lower than in the virgin rat liver during early lactation and increased by 2-fold in liver of mid-lactating rats. Weaning of mid-lactating rats for 24 h resulted in no change in A and C activities but after 48 h weaning they were significantly lower than those in livers from suckled mothers. 4. The I activity followed a similar pattern of changes as the A and C activities during pregnancy and lactation such that, although the I/A and I/C activity ratios tended to be lower during late pregnancy and early lactation, there were no significant changes in I/A and I/C ratios between lactating and virgin animals. However, these ratios were significantly higher in liver from fed 24 h-weaned animals. 5. Starvation (24 h) resulted in a marked decrease in I activity for all animals studied except early-lactating rats. This was due to a combination of a decrease in the concentration of acetyl-CoA carboxylase in liver of starved animals (A and C activities) and a decrease in the fraction of the enzyme in the active form (lower I/C and I/A ratios). The relative importance of the two forms of regulation in mediating the starvation-induced fall in I activity was about equal in livers of virgin, pregnant and lactating animals. However, the decrease in I/A and I/C ratios was of dominating importance in livers of weaned animals. The A/C activity ratios were the same for livers from all animals studied. 6. The maximal activity of fatty acid synthase was also measured in livers and was highly and positively correlated with the A and C activities of acetyl-CoA carboxylase, suggesting that the concentrations of the two enzymes in the liver were controlled coordinately. 7. It is suggested that the lack of correlation between plasma insulin levels and rates of lipogenesis in the transition from the virgin to the lactating state may be explained by different effects of insulin and prolactin on the concentration of acetyl-CoA carboxylase in the liver and on the fraction of the enzyme in the active form.
Full text
PDF


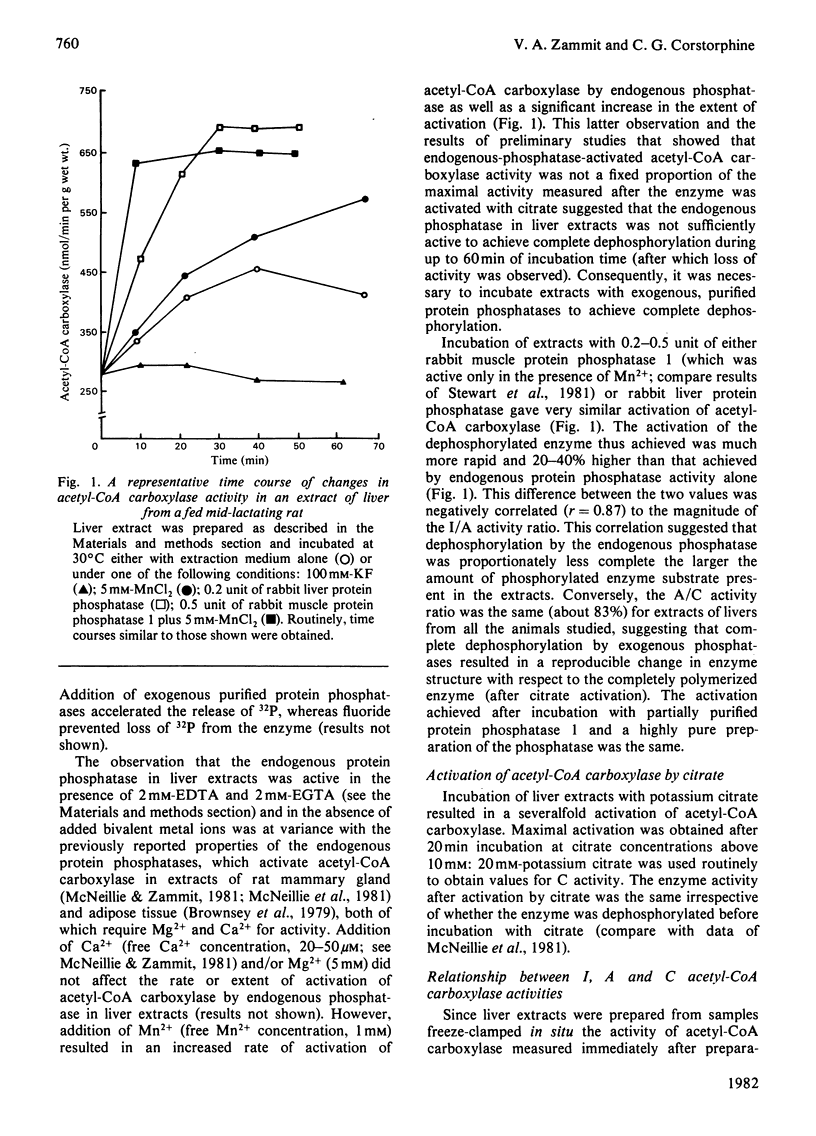



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLMANN D. W., HUBBARD D. D., GIBSON D. M. FATTY ACID SYNTHESIS DURING FAT-FREE REFEEDING OF STARVED RATS. J Lipid Res. 1965 Jan;6:63–74. [PubMed] [Google Scholar]
- Agius L., Robinson A. M., Girard J. R., Williamson D. H. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: a possible regulatory role of prolactin. Biochem J. 1979 Jun 15;180(3):689–692. doi: 10.1042/bj1800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred J. B., Roehrig K. L. Heat activation of rat liver acetyl-CoA carboxylase in vitro. J Biol Chem. 1978 Jul 25;253(14):4826–4829. [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., Denton R. M., Jeanrenaud B. Stimulation of hepatic lipogenesis and acetyl-coenzyme A carboxylase by vasopressin. Biochem J. 1981 Sep 15;198(3):485–490. doi: 10.1042/bj1980485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt H., Capulong Z. L., Lee E. Y. Purification and properties of rabbit liver phosphorylase phosphatase. J Biol Chem. 1975 Oct 25;250(20):8038–8044. [PubMed] [Google Scholar]
- Brownsey R. W., Hughes W. A., Denton R. M. Adrenaline and the regulation of acetyl-coenzyme A carboxylase in rat epididymal adipose tissue. Inactivation of the enzyme is associated with phosphorylation and can be reversed on dephosphorylation. Biochem J. 1979 Oct 15;184(1):23–32. doi: 10.1042/bj1840023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R. W., Hughes W. A., Denton R. M. Demonstration of the phosphorylation of acetyl-coenzyme A carboxylase within intact rat epididymal fat-cells. Biochem J. 1977 Dec 15;168(3):441–445. doi: 10.1042/bj1680441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M. C., Nepokroeff C. M., Lakshmanan M. R., Porter J. W. Effect of dietary change on the rates of synthesis and degradation of rat liver fatty acid synthetase. Arch Biochem Biophys. 1972 Oct;152(2):619–630. doi: 10.1016/0003-9861(72)90258-5. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Denton R., Bridges B., Brownsey R., Evans G., Hughes W., Stansbie D. Regulation of the conversion of glucose into fat in white adipose tissue by insulin [proceedings]. Biochem Soc Trans. 1977;5(4):894–900. doi: 10.1042/bst0050894. [DOI] [PubMed] [Google Scholar]
- Geelen M. J., Beynen A. C., Christiansen R. Z., Lepreau-Jose M. J., Gibson D. M. Short-term effects of insulin and glucagon on lipid synthesis in isolated rat hepatocytes. Covariance of acetyl-CoA carboxylase activity and the rate of 3H2O incorporation into fatty acids. FEBS Lett. 1978 Nov 15;95(2):326–330. doi: 10.1016/0014-5793(78)81022-9. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Kim K. H. Control of acetyl-CoA carboxylase by covalent modification. Mol Cell Biochem. 1979 Dec 14;28(1-3):27–43. doi: 10.1007/BF00223358. [DOI] [PubMed] [Google Scholar]
- Krakower G. R., Kim K. H. Heat activation of rat epididymal fat tissue acetyl-coa carboxylase is due to dephosphorylation by its endogenous phosphatase. Lipids. 1980 Dec;15(12):1067–1070. doi: 10.1007/BF02534326. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Moss J., Polakis S. E. Acetyl coenzyme A carboxylase. Curr Top Cell Regul. 1974;8(0):139–195. [PubMed] [Google Scholar]
- Lane M. D., Watkins P. A., Meredith M. J. Hormonal regulation of acetyl-CoA carboxylase activity in the liver cell. CRC Crit Rev Biochem. 1979 Dec;7(2):121–141. doi: 10.3109/10409237909105429. [DOI] [PubMed] [Google Scholar]
- McNeillie E. M., Clegg R. A., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of incubation with Ca2+, Mg2+ and ATP on enzyme activity in tissue extracts. Biochem J. 1981 Dec 15;200(3):639–644. doi: 10.1042/bj2000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeillie E. M., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of starvation and of insulin and prolactin deficiency on the fraction of the enzyme in the active form in vivo. Biochem J. 1982 Apr 15;204(1):273–280. doi: 10.1042/bj2040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto Y., Gibson D. M. Selective dampening of lipogenic enzymes of liver by exogenous polyunsaturated fatty acids. Biochem Biophys Res Commun. 1970 Jan 6;38(1):9–15. doi: 10.1016/0006-291x(70)91076-4. [DOI] [PubMed] [Google Scholar]
- Nepokroeff C. M., Lakshmanan M. R., Ness G. C., Muesing R. A., Kleinsek D. A., Porter J. W. Coordinate control of rat liver lipogenic enzymes by insulin. Arch Biochem Biophys. 1974 Jun;162(2):340–344. doi: 10.1016/0003-9861(74)90191-x. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. W. The effects of pregnancy and lactation on the activities in rat liver of some enzymes associated with glucose metabolism. Biochim Biophys Acta. 1975 Nov 10;411(1):22–29. doi: 10.1016/0304-4165(75)90281-0. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. A., Hemmings B. A., Cohen P., Goris J., Merlevede W. The MgATP-dependent protein phosphatase and protein phosphatase 1 have identical substrate specificities. Eur J Biochem. 1981 Mar 16;115(1):197–205. doi: 10.1111/j.1432-1033.1981.tb06217.x. [DOI] [PubMed] [Google Scholar]
- Swanson R. F., Curry W. M., Anker H. S. The activation of rat liver acetyl-CoA carboxylase by trypsin. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1243–1248. doi: 10.1073/pnas.58.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Moriarity D., Martin D. B. Regulation of hepatic acetyl coenzyme A carboxylase by insulin and glucagon. J Biol Chem. 1979 Jul 25;254(14):6644–6649. [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J. 1981 Jul 15;198(1):75–83. doi: 10.1042/bj1980075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. The effect of glucagon treatment and starvation of virgin and lactating rats on the rates of oxidation of octanoyl-L-carnitine and octanoate by isolated liver mitochondria. Biochem J. 1980 Aug 15;190(2):293–300. doi: 10.1042/bj1900293. [DOI] [PMC free article] [PubMed] [Google Scholar]


