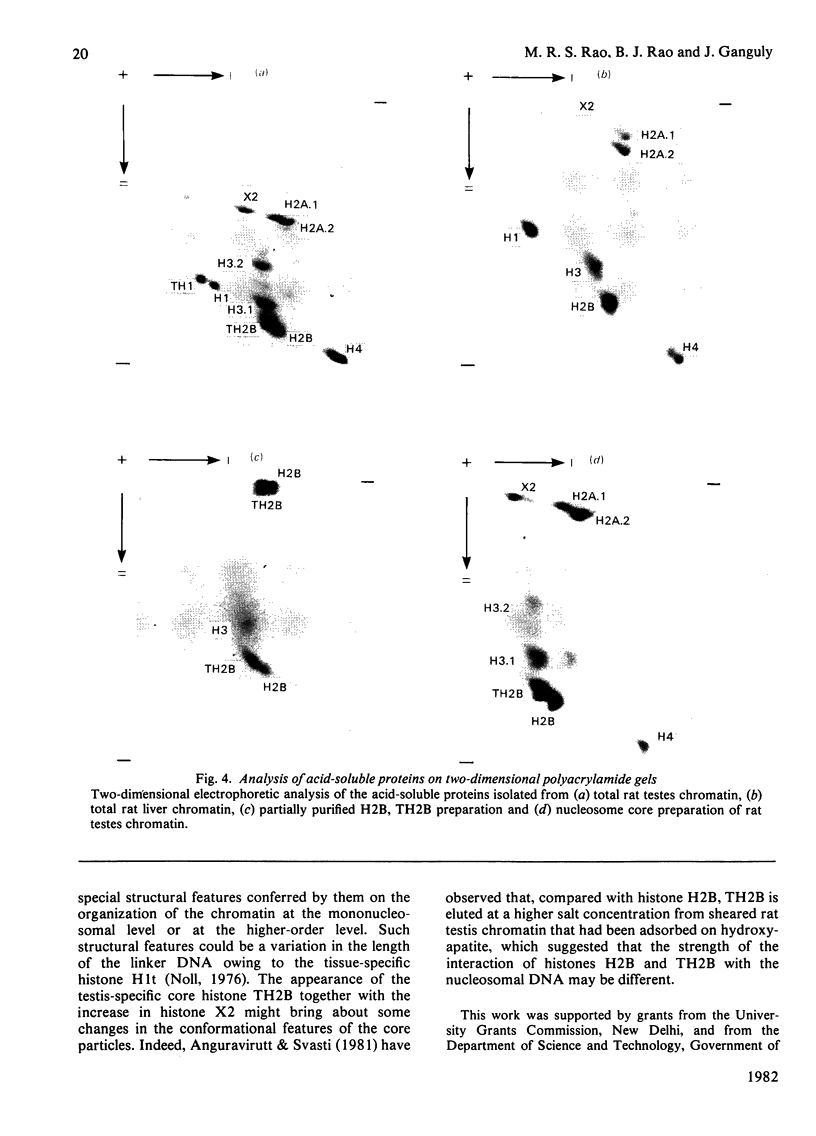
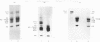Abstract
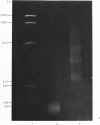
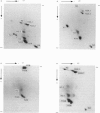
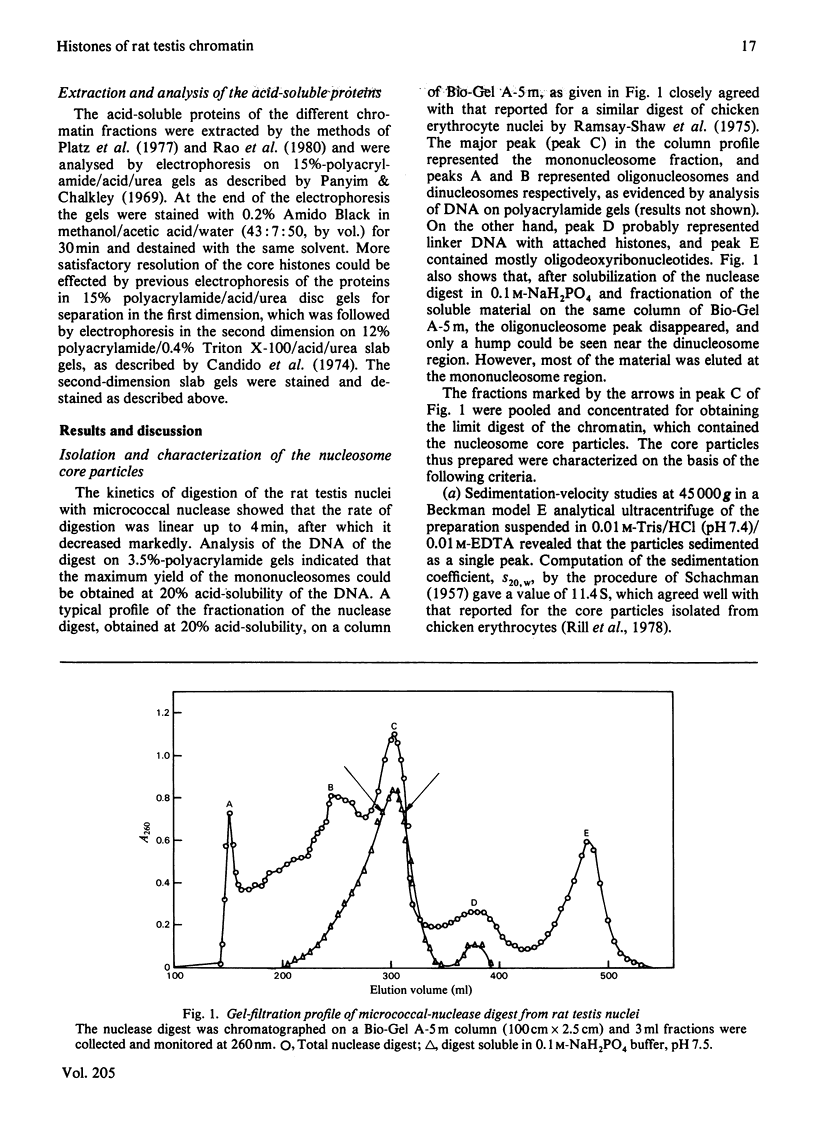
Nucleosome core particles and oligonucleosomes were isolated by digesting rat testis nuclei with micrococcal nuclease to 20% acid-solubility, followed by fractionation of the digest on a Bio-Gel A-5m column. The core particles thus isolated were characterized on the basis of their DNA length of 151 +/- 5 base-pairs and sedimentation coefficient of 11.4S. Analysis of the acid-soluble proteins of the core particles indicated that histones TH2B and X2 are constituents of the core particles, in addition to the somatic histones H2A, H2B, H3 and H4. The acid-soluble proteins of the oligonucleosomes comprised all the histones, including both the somatic (H1, H2A, H2B, H3, H4 and X2) and the testis-specific ones (TH1 and TH2B). It was also observed that histones TH1 and H1 are absent from the core particles and were readily extracted from the chromatin by 0.6 M-NaCl, which indicated that both of them are bound to the linker DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Anguravirutt S., Svasti J. A new procedure for the purification of rat testis-specific histone TH2B involving affinity-related chromatography. Arch Biochem Biophys. 1981 Aug;210(1):412–416. doi: 10.1016/0003-9861(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Branson R. E., Grimes S. R., Jr, Yonuschot G., Irvin J. L. The histones of rat testis. Arch Biochem Biophys. 1975 Jun;168(2):403–412. doi: 10.1016/0003-9861(75)90269-6. [DOI] [PubMed] [Google Scholar]
- Böhm E. L., Strickland W. N., Strickland M., Thwaits B. H., van der Westhuizen D. R., von Holt C. Purification of the five main calf thymus histone fractions by gel exclusion chromatography. FEBS Lett. 1973 Aug 15;34(2):217–221. doi: 10.1016/0014-5793(73)80797-5. [DOI] [PubMed] [Google Scholar]
- Dooher G. B., Bennett D. Fine structural observations on the development of the sperm head in the mouse. Am J Anat. 1973 Mar;136(3):339–361. doi: 10.1002/aja.1001360307. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Mathew C. G., Wright C. A., Venkov C. D., Johns E. W. Analysis of the high mobility group proteins associated with salt-soluble nucleosomes. Nucleic Acids Res. 1979 Dec 11;7(7):1815–1835. doi: 10.1093/nar/7.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Chae C. B., Irvin J. L. Effects of age and hypophysectomy upon relative proportions of various histones in rat testis. Biochem Biophys Res Commun. 1975 Jan 2;64(3):911–917. doi: 10.1016/0006-291x(75)90134-5. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed Proc. 1978 Sep;37(11):2512–2516. [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975 May;65(2):258–270. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Kumaroo K. K., Irvin J. L. Isolation of histone TH1-xB from rat testis. Biochem Biophys Res Commun. 1980 May 14;94(1):49–54. doi: 10.1016/s0006-291x(80)80185-9. [DOI] [PubMed] [Google Scholar]
- Kumaroo K. K., Jahnke G., Irvin J. L. Changes in basic chromosomal proteins during spermatogenesis in the mature rat. Arch Biochem Biophys. 1975 Jun;168(2):413–424. doi: 10.1016/0003-9861(75)90270-2. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Precise location of DNase I cutting sites in the nucleosome core determined by high resolution gel electrophoresis. Nucleic Acids Res. 1979 Jan;6(1):41–56. doi: 10.1093/nar/6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R., Burch J. B., Kunkel G. Semihistone protein A24 replaces H2A as an integral component of the nucleosome histone core. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1030–1034. doi: 10.1073/pnas.76.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marushige Y., Marushige K. Transformation of sperm histone during formation and maturation of rat spermatozoa. J Biol Chem. 1975 Jan 10;250(1):39–45. [PubMed] [Google Scholar]
- Meistrich M. L., Brock W. A., Grimes S. R., Platz R. D., Hnilica L. S. Nuclear protein transitions during spermatogenesis. Fed Proc. 1978 Sep;37(11):2522–2525. [PubMed] [Google Scholar]
- Meistrich M. L., Reid B. O., Barcellona W. J. Changes sperm culei during sperimogensis and epidymal maturation. Exp Cell Res. 1976 Apr;99(1):72–78. doi: 10.1016/0014-4827(76)90681-9. [DOI] [PubMed] [Google Scholar]
- Noll M. Differences and similarities in chromatin structure of Neurospora crassa and higher eucaryotes. Cell. 1976 Jul;8(3):349–355. doi: 10.1016/0092-8674(76)90146-x. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Platz R. D., Grimes S. R., Meistrich M. L., Hnilica L. S. Changes in nuclear proteins of rat testis cells separated by velocity sedimentation. J Biol Chem. 1975 Aug 10;250(15):5791–5800. [PubMed] [Google Scholar]
- Platz R. D., Meistrich M. L., Grimes S. R., Jr Low-molecular-weight basic proteins in spermatids. Methods Cell Biol. 1977;16:297–316. doi: 10.1016/s0091-679x(08)60107-7. [DOI] [PubMed] [Google Scholar]
- Rao M. R., Prasad V. R., Padmanaban G., Ganguly J. Isolation and characterization of binding proteins for retinol from the cytosol, nucleosol and chromatin of the oviduct magnum of laying hens. Biochem J. 1979 Dec 1;183(3):501–506. doi: 10.1042/bj1830501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. R., Singh J., Ganguly J. Effect of deprivation of vitamin A on the basic proteins of the nuclei of rat testes. Biochem Biophys Res Commun. 1980 May 14;94(1):1–8. doi: 10.1016/s0006-291x(80)80178-1. [DOI] [PubMed] [Google Scholar]
- Rill R. L., Shaw B. R., Van Holde K. E. Isolation and characterization of chromatin subunits. Methods Cell Biol. 1978;18:69–103. doi: 10.1016/s0091-679x(08)60134-x. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Kistler W. S. Isolation and characterization of rat testis H1t. An H1 histone variant associated with spermatogenesis. J Biol Chem. 1980 Jun 25;255(12):5949–5954. [PubMed] [Google Scholar]
- Shaw B. R., Corden J. L., Sahasrabuddhe C. G., Van Holde K. E. Chromatographic separation of chromatin subunits. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1193–1198. doi: 10.1016/s0006-291x(74)80410-9. [DOI] [PubMed] [Google Scholar]
- Shires A., Carpenter M. P., Chalkley R. A cysteine-containing H2B-like histone found in mature mammalian testis. J Biol Chem. 1976 Jul 10;251(13):4155–4158. [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker R. G., Blanchard B. J., Ingram V. M. A simple purification procedure for monomeric nucleosomes. Anal Biochem. 1979 Jan 15;92(2):420–425. doi: 10.1016/0003-2697(79)90680-8. [DOI] [PubMed] [Google Scholar]