Abstract
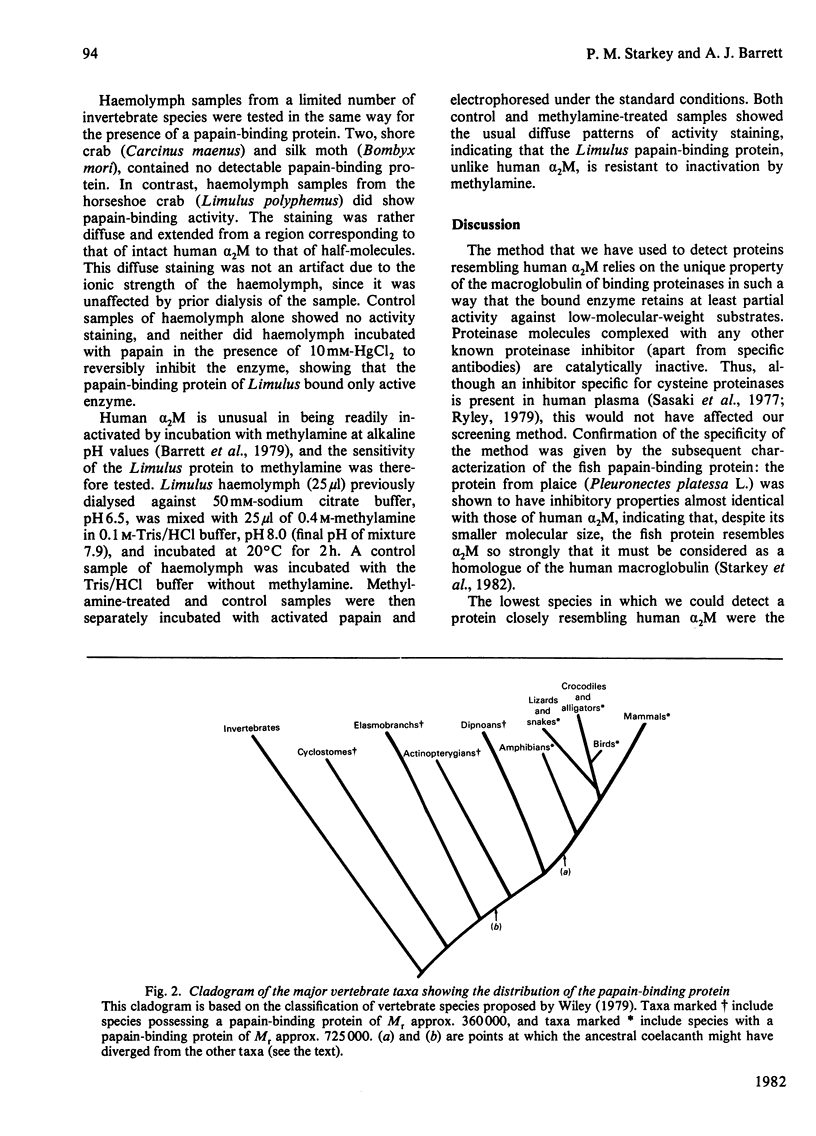
Plasma or serum samples from a large number of vertebrate species were screened for the presence of a papain-binding protein resembling human alph a 2-macroglobulin (alpha 2M). The screening method depended on the unique property of alpha 2M of binding proteinases in such a way that the enzyme retains partial activity against low-molecular-weight substrates. A papain-binding protein was detected in serum from members of all the major vertebrate taxa. In mammals, birds, reptiles and amphibians the protein had an Mr similar to that of human alpha 2M (725 000), but in fish, including dipnoans, actinopterygians, elasmobranchs and cyclostomes, the papain-binding protein was of Mr about 360 000. Of the invertebrate species tested, all of which were arthropods, two were negative, but the horseshoe crab, an arachnid, did possess a papain-binding protein, although this was heterogeneous in electrophoresis and differed from alpha 2M in resisting inactivation by methylamine. From the results, and a detailed study of the properties of the fish papain-binding protein described in an accompanying paper [Starkey, Fletcher & Barrett (1982) Biochem. J. 205, 97-104], it seems that alpha 2M first appeared in an ancestor of all modern vertebrates as a protein of Mr 360 000 and that the larger macroglobulin (Mr 725 000) first appeared in an ancestor of the tetrapods.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armand Y. J., Guinand S. Composition et structure de L'alpha-2-macroglobuline isolée du serum de porc. Biochim Biophys Acta. 1967 Feb 21;133(2):289–300. [PubMed] [Google Scholar]
- Barrett A. J. An improved color reagent for use in Barrett's assay of Cathepsin B. Anal Biochem. 1976 Nov;76(50):374–376. doi: 10.1016/0003-2697(76)90298-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne B. H., Dray S., Knight K. L. Immunological relationships of serum alpha-macroglobulins in the human, rat, and rabbit. Proc Soc Exp Biol Med. 1971 Nov;138(2):531–535. doi: 10.3181/00379727-138-35933. [DOI] [PubMed] [Google Scholar]
- Ganrot K. 2 -acute phase globulin in rat serum. Purification, determination and interaction with trypsin. Biochim Biophys Acta. 1973 Jan 25;295(1):245–251. doi: 10.1016/0005-2795(73)90091-3. [DOI] [PubMed] [Google Scholar]
- Got R., Mouray H., Moretti J. Etude biochimique de l'alpha 1-macroglobuline du Sérum de lapin. I. Préparation et propriétés physico-chimiques. Biochim Biophys Acta. 1965 Sep 13;107(2):278–285. [PubMed] [Google Scholar]
- Greene N. D., Damian R. T., Hubbard W. J. The identification of alpha-2-macroglobulin in the mouse. Biochim Biophys Acta. 1971 Jun 29;236(3):659–663. doi: 10.1016/0005-2795(71)90252-2. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Creeth J. M., Kekwick R. A. Thio reduction of human 2 -macroglobulin. The subunit structure. Biochem J. 1972 Mar;127(1):187–197. doi: 10.1042/bj1270187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne M., Raynaud M. Préparation et propriétés de l'alpha2-macroglobuline de cheval. Ann Inst Pasteur (Paris) 1970 Jul;119(1):27–49. [PubMed] [Google Scholar]
- Lebreton de Vonne T., Mouray H. Isolement d'alpha 1 M et d'alpha-2 M du lapin et leur influence sur l'activité enzymatique de la trypsine. C R Acad Sci Hebd Seances Acad Sci D. 1968 Mar 4;266(10):1076–1079. [PubMed] [Google Scholar]
- Nagasawa S., Han B. H., Sugihara H., Suzuki T. Studies on alpha 2-macroglobulin in bovine plasma. II. Interaction of alpha2-macroglobulin and trypsin. J Biochem. 1970 Jun;67(6):821–832. doi: 10.1093/oxfordjournals.jbchem.a129314. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Emeis J. J., Hemmink J. Purification and properties of rat alpha 2 acute-phase macroglobulin. Biochim Biophys Acta. 1979 Sep 29;580(1):129–139. doi: 10.1016/0005-2795(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Ohlsson K. Isolation and partial characterization of two related trypsin binding alpha-macroglobulins of dog plasma. Biochim Biophys Acta. 1971 Apr 27;236(1):84–91. doi: 10.1016/0005-2795(71)90153-x. [DOI] [PubMed] [Google Scholar]
- PICARD J. J., HEREMANS J. F. Studies on alpha2-macroblobulin. I. A method for the isolation of rabbit alpha2-macroglobulin. Biochim Biophys Acta. 1963 Jun 4;71:554–561. doi: 10.1016/0006-3002(63)91127-2. [DOI] [PubMed] [Google Scholar]
- Pepper D. S. The isolation and properties of gamma inhibitor: an equine alpha 2-macroglobulin sub-fraction. Biochim Biophys Acta. 1968 Mar 11;156(2):327–339. doi: 10.1016/0304-4165(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Picard J. J., Vandebroek G., Heremans J. F., Defossé G. Studies on alpha-2-macroglobulin. 3. Isolation and properties of Erinaceus alpha-2-macroglobulin. Biochim Biophys Acta. 1966 Mar 28;117(1):111–114. doi: 10.1016/0304-4165(66)90158-9. [DOI] [PubMed] [Google Scholar]
- Ryley H. C. Isolation and partial characterisation of a thiol proteinase inhibitor from human plasma. Biochem Biophys Res Commun. 1979 Aug 13;89(3):871–878. doi: 10.1016/0006-291x(79)91859-x. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Minakata K., Yamamoto H., Niwa M., Kato T., Ito N. A new serum component which specifically inhibits thiol proteinases. Biochem Biophys Res Commun. 1977 Jun 6;76(3):917–924. doi: 10.1016/0006-291x(77)91589-3. [DOI] [PubMed] [Google Scholar]
- Shortridge K. F., Belyavin G., Bidwell D. E. Human serum alpha2-macroglobulin: immunological cross-reactivity with alpha2-macroglobulins in vertebrate sera. Comp Biochem Physiol A Comp Physiol. 1976;54(3):319–321. doi: 10.1016/s0300-9629(76)80119-3. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Inhibition by alpha-macroglobulin and other serum proteins. Biochem J. 1973 Apr;131(4):823–831. doi: 10.1042/bj1310823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Fletcher T. C., Barrett A. J. Evolution of alpha 2-macroglobulin. The purification and characterization of a protein homologous with human alpha 2-macroglobulin from plaice (Pleuronectes platessa L.) plasma. Biochem J. 1982 Jul 1;205(1):97–104. doi: 10.1042/bj2050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuru D., Tomimatsu M., Fujiwara K., Kawahara K. A neutral subtilopeptidase inhibitor from porcine serum some evidence for alpha2-macroglobulin. J Biochem. 1975 Jun;77(6):1305–1312. [PubMed] [Google Scholar]



