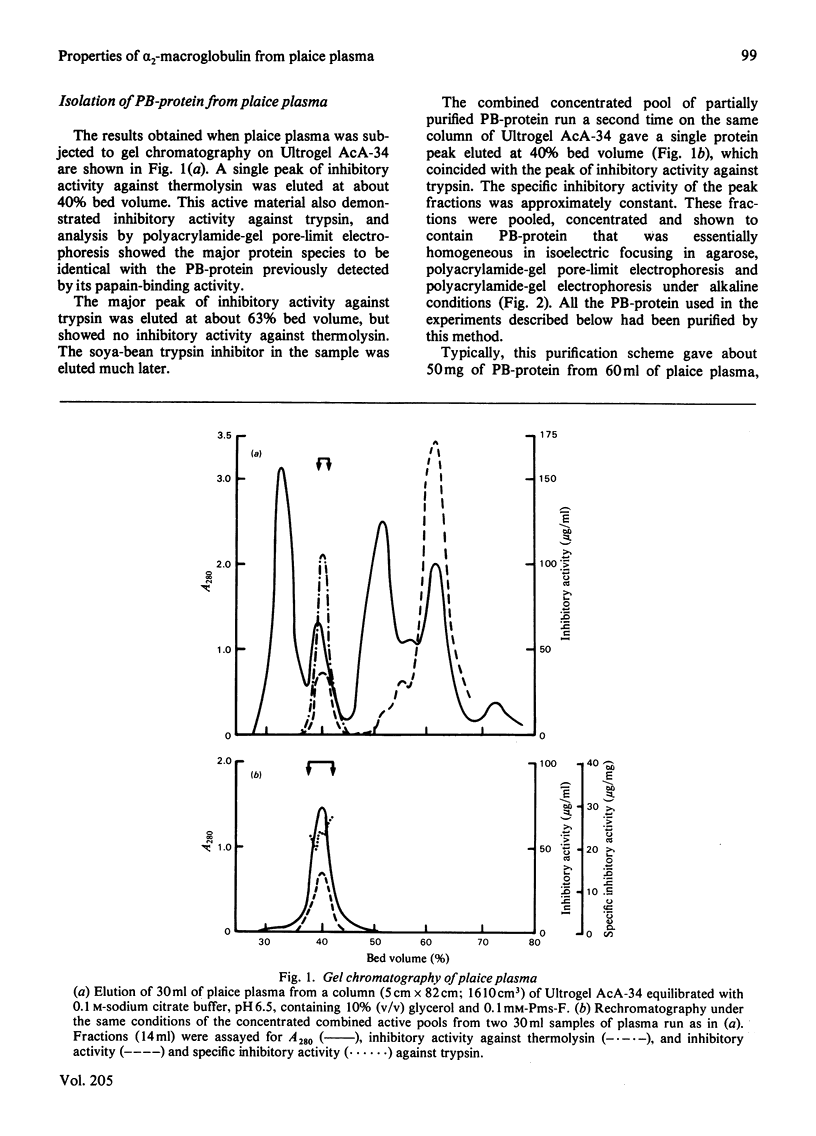
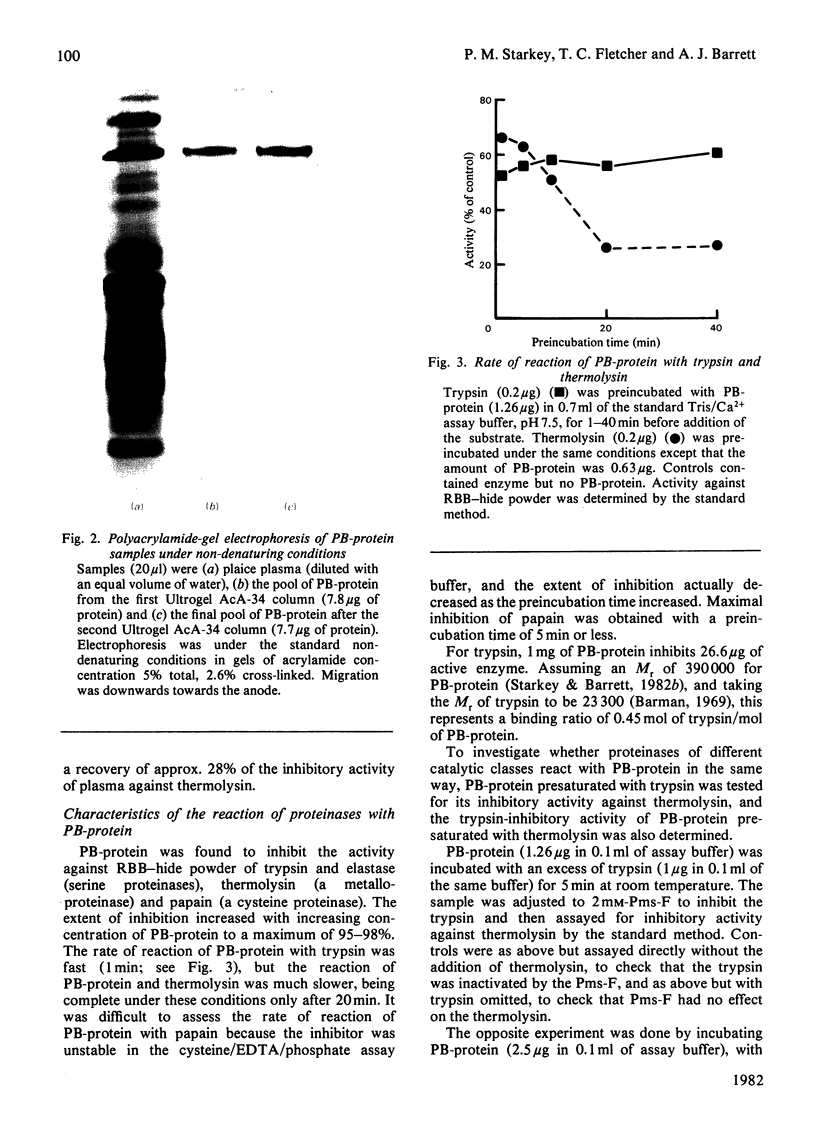
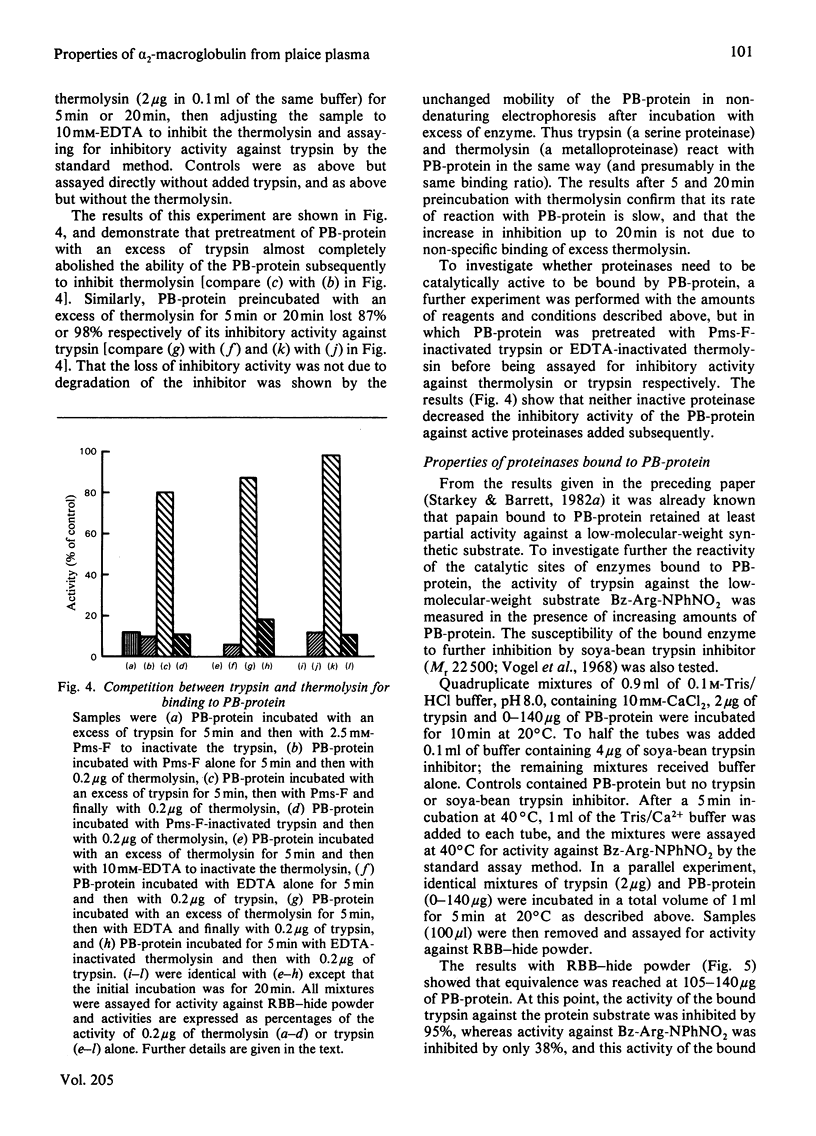
Abstract
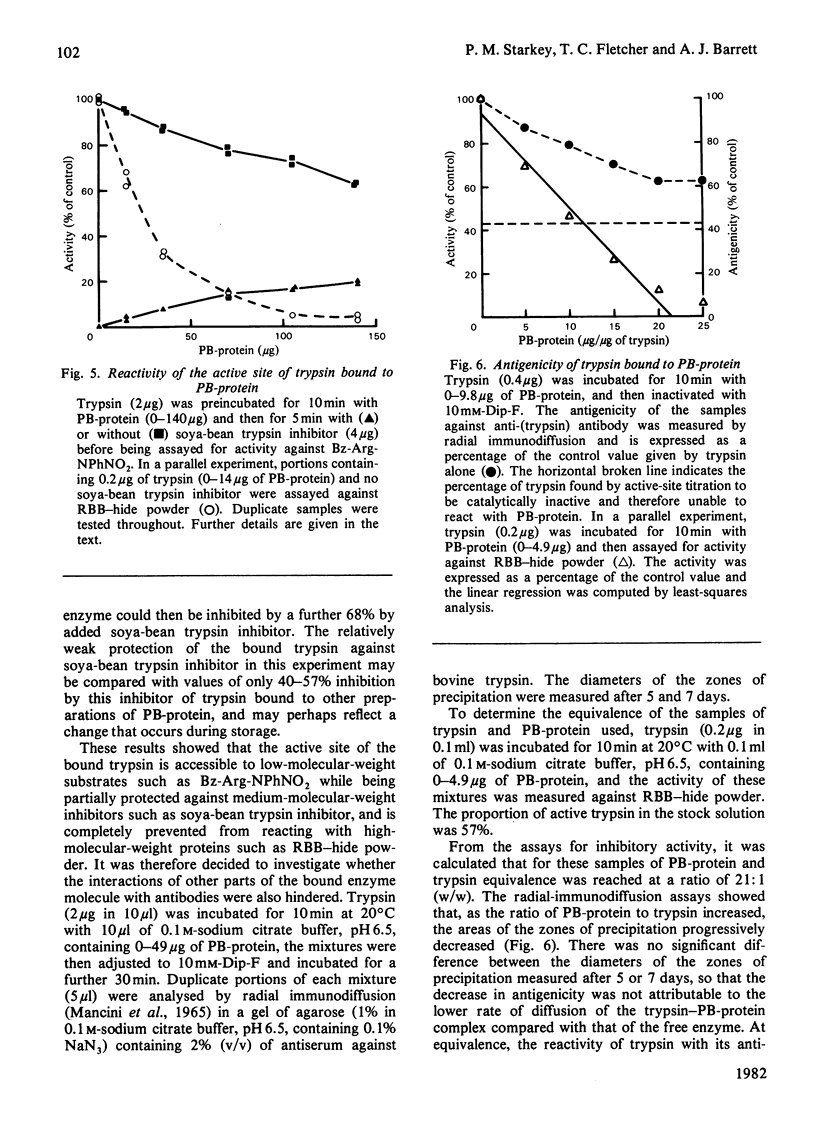
A papain-binding protein (PB-protein) was purified to homogeneity from the plasma of plaice (Pleuronectes platessa L.). PB-protein inhibited the activity of trypsin and pancreatic elastase (serine proteinases), thermolysin (a metalloproteinase) and papain (a cysteine proteinase). Presaturation of PB-protein with trypsin prevented the subsequent inhibition of thermolysin, and vice versa. Only catalytically active endopeptidases were bound by PB-protein. The catalytic activity of trypsin bound by PB-protein was inhibited by 95% against an insoluble protein substrate, but only by 38% against a low-molecular-weight synthetic substrate. The remaining activity of the bound trypsin was partially protected against further inhibition by soya-bean trypsin inhibitor. Trypsin bound by PB-protein showed a decrease of 67% in its reactivity with antibodies. The inhibitory activity of PB-protein was inactivated at pH 8.0 by methylamine (0.2M) or dithiothreitol (1 mM). The inhibition of proteinases by plaice PB-protein shows the distinctive characteristics of inhibition by human alpha 2-macroglobulin, and it is concluded that the plaice protein is a homologue of the human macroglobulin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Gordon J., Whitehead H. R., Wormall A. The Action of Ammonia on Complement. The Fourth Component. Biochem J. 1926;20(5):1028–1035. doi: 10.1042/bj0201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H., PILLEMER L. The multiplicity of plasmin inhibitors in human serum, demonstrated by the effect of primary amino compounds. Bull Johns Hopkins Hosp. 1954 Apr;94(4):169–179. [PubMed] [Google Scholar]
- Rosén A., Ek K., Aman P. Agarose isoelectric focusing of native human immunoglobulin M and alpha 2-macroglobulin. J Immunol Methods. 1979;28(1-2):1–11. doi: 10.1016/0022-1759(79)90322-3. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Sim E. Autolytic fragmentation of complement components C3 and C4 under denaturing conditions, a property shared with alpha 2-macroglobulin. Biochem J. 1981 Jan 1;193(1):129–141. doi: 10.1042/bj1930129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Evolution of alpha 2-macroglobulin. The demonstration in a variety of vertebrate species of a protein resembling human alpha 2-macroglobulin. Biochem J. 1982 Jul 1;205(1):91–95. doi: 10.1042/bj2050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Evolution of alpha 2-macroglobulin. The structure of a protein homologous with human alpha 2-macroglobulin from plaice (Pleuronectes platessa L.) plasma. Biochem J. 1982 Jul 1;205(1):105–115. doi: 10.1042/bj2050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C., Barrett A. J., Starkey P. M. The interaction of alpha2-macroglobulin with proteinases. Binding and inhibition of mammalian collagenases and other metal proteinases. Biochem J. 1974 May;139(2):359–368. doi: 10.1042/bj1390359. [DOI] [PMC free article] [PubMed] [Google Scholar]