Abstract
Chronic otitis media with effusion (COME) is a common cause of hearing loss in children and adults. Laryngopharyngeal reflux (LPR) is often overlooked in the clinical management of COME complicated by LPR. This study aimed to investigate the presence and concentration of trypsin and pepsin in the middle ear effusion (MEE), as well as the recurrence rate of otitis media with effusion (OME) in COME patients with trypsin-/pepsin-positive MEE after acid-suppressive treatment (AST). In this study, trypsin and pepsin were found in the MEE of COME patients. Follow-up results showed that the recurrence rate of OME within 1 year was significantly lower in the AST group than in the non-AST group. Taken together, patients with COME can be offered pepsin detection of MEE, and if the pepsin test is positive, acid-suppressing drugs such as proton pump inhibitors could be recommended to prevent the recurrence of OME.
Keywords: Trypsin, Pepsin, Chronic otitis media with effusion, Laryngopharyngeal reflux
1. Introduction
Otitis media with effusion (OME) is a non-purulent inflammatory disease of the middle ear, mainly characterized by conductive hearing loss and effusion in the middle ear. Research has shown that around 90 % of preschool children have OME [1]. If not properly treated, it can lead to chronic OME (COME) with complications such as eardrum invagination, tympanic sclerosis, adhesive otitis media, permanent hearing loss and even speech and language impairment in children [2]. COME is the most common cause of acquired hearing loss in children [3].
The pathogenesis of OME is not fully understood. Mainstream theories include negative pressure in the middle ear [4], infection [5,6] and allergy [7]. Many studies have shown that there is a correlation between laryngopharyngeal reflux (LPR) and OME [8,9], and that LPR can prolong the course of OME [10,11]. In recent years, researchers have detected pepsin in the middle ear effusion (MEE) of OME patients, and the concentration is 100 times higher than that in serum [10,11]. The middle ear mucosa does not have the function of secreting gastric protease. It has been speculated that pepsin in the MEE may originate from the digestive fluid or gaseous digestive fluid of LPR through the eustachian tube.
LPR is a chronic condition with mucosal injury caused by abnormal reflux of gastric contents into the pharynx, larynx and upper airways [12]. The reflux of LPR includes pepsin, gastric acid, bile acid and trypsin. However, few studies have detected the presence of trypsin with stronger digestion in MEE. LPR reflux tends to trigger an inflammatory cascade response [13], and as the reflux is transported into the airways, it is buffered into weakly acidic or non-acidic substances. The airway mucosa is damaged by these non-acidic components, leading to frequent respiratory symptoms such as throat clearing, coughing and asthma [14]. Children with poor expressive language skills are often overlooked by family members when LPR symptoms occur. The Dx-pH monitoring system is currently the objective standard for LPR diagnosis [15]. It is an invasive test that is relatively expensive and causes significant discomfort during the examination process. Some patients cannot tolerate this examination. The Reflux Symptom Index (RSI) is the most commonly used diagnostic and assessment method for the clinical diagnosis of LPR due to its simplicity and accuracy [16]. Research has shown that pepsin measurement in saliva could be an alternative tool to assist in the diagnosis of LPR [17].
The characteristics of MEE can vary greatly [18]. Some MEE is fluid like water, with a pale-yellow color. Some are sticky like glue. In this study, we used the RSI scale and MEE trypsin and pepsin detection to diagnose whether patients with OME had LPR. The collected MEE was divided into serous and mucous groups and pH tests were performed on both. We performed Western blotting (WB) to detect the presence of trypsin and pepsin in the MEE. In those with a positive WB, an enzyme-linked immunosorbent assay (ELISA) is conducted to determine the concentration of trypsin and pepsin. Individuals with pepsin or trypsin positive were randomly divided into two groups, one receiving acid-suppressive treatment (AST, proton pump inhibitors for 8 weeks) and the other being followed. 1 year later, the recurrence rate of OME was calculated.
2. Materials and methods
2.1. Human subjects
We conducted a prospective cohort study of patients admitted to the Department of Otorhinolaryngology-Head and Neck Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China, with a diagnosis of unilateral COME from January 2018 to December 2023.
All participants underwent the following four parts before enrolment.
-
①
General information (age, sex, recent medication, etc.) and completion of the RSI scale.
-
②
Audiological examination: pure tone audiometry (adult) or auditory brainstem response (ABR) (child), acoustic immittance measurement, MEE collection and MEE bacterial culture.
-
③
Endoscopic photography of the tympanic membrane and nasopharynx.
-
④
Lateral skull film.
Inclusion criteria for the experimental group: duration of OME more than 3 months; conductive hearing loss on audiogram (adult)/ABR (child); B-type curve on tympanogram; evidence of MEE on otoscopy; negative bacterial culture of MEE.
Exclusion criteria for the experimental group: Patients were excluded if they had any concomitant disease or trauma contributing to hearing loss, such as chronic purulent otitis media, tympanic membrane perforation, adenoid hypertrophy in children or residual adenoids in adults (adenoidal-nasopharyngeal (A/N) ratio obtained from lateral skull film <0.6) [19], nasopharyngeal or ear tumors, or had received AST within 2 weeks, or had a positive bacterial culture of the MEE, or had a non-B-type curve on the tympanogram, or had used ototoxic medications.
A total of 270 patients (150 adults and 120 children) and 50 healthy subjects were included in this study. There was no statistically significant difference in age or gender (P > 0.05).
2.2. Materials
Pepsin and trypsin antibodies were bought from Abcam plc (Shanghai, China). The pepsin and trypsin Elisa kits were purchased from Cloud-Clone Corp (Wuhan, China).
2.3. MEE collection and pH detection
Child group: After general anesthesia, the external acoustic meatus was rinsed and disinfected with iodophor and then rinsed three times with a saline solution. An endoscopic incision of approximately 2–3 mm was made in the anterior inferior quadrant of the tympanic membrane. A sterile suction device was used to slowly aspirate the effusion and a portion was quickly removed for bacterial culture and pH testing. The remaining portion was placed in a 1.5 mL sterile tube and stored at −80 °C for future use.
Adult group: The external acoustic meatus was rinsed and disinfected with iodophor and then rinsed three times with a saline solution. A thin cotton swab soaked in 1 % tetracaine should be gently applied to the skin of the external ear canal and tympanic membrane for 10 min. Puncture the anterior lower quadrant of the tympanic membrane with a needle. Fix the needle, slowly aspirate the effusion and quickly collect a portion for bacterial culture and pH testing. The remaining portion was placed in a 1.5 mL sterile tube and stored at −80 °C for future use.
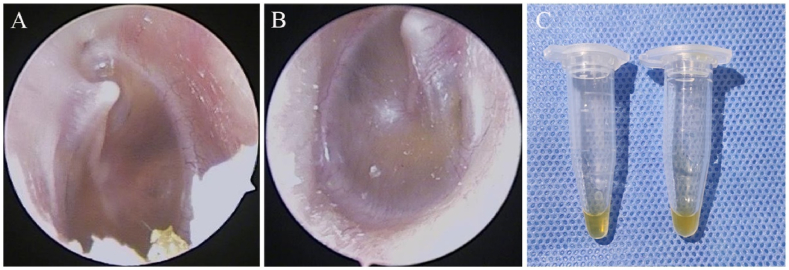
The collected MEE were divided into two groups: serous and mucous, based on the characteristics of the MEE. If the MEE is clear, watery and easy to aspirate, it is classified as serous MEE (Fig. 1A). If the MEE is viscous, gelatinous and difficult to aspirate, it is classified as mucous MEE (Fig. 1B). They were all labelled and collected in 1.5 mL sterile tubes (Fig. 1C).
Fig. 1.
Otoscopic signs and effusion samples of serous and mucous groups. (A) Representative endoscopic sign of the serous group. (B) Representative endoscopic sign of the mucous group. (C) On the left is an effusion sample from the serous group and on the right is an effusion sample from the mucous group.
Control group: A nasopharyngeal swab was passed through the nasal meatus into the nasopharynx using an electronic nasopharyngoscope. The pharyngeal opening of the Eustachian tube was wiped with the swab and washed with 1 mL of physiological saline in a 1.5 mL sterile tube and stored at −80 °C for future use.
2.4. RSI scale
All participants must complete the RSI scale under the guidance of the same professional ENT physician. Older children can complete the RSI scale with the help of their parents, while younger children or those who are uncooperative can have their parents complete the RSI scale. The severity of each symptom on the RSI scale was self-rated by the patient, and to minimize the influence of subjective factors. An RSI score greater than 13 was considered positive.
2.5. WB experiments
Centrifuge tube pre-cooling, after thawing the sample, take a portion of the sample into the pre-cooled centrifuge tube, add protease inhibitors and lysis solution and lyse on ice for half an hour. After centrifugation (12000 r/min, 4 °C, 5 min), transfer the supernatant to a new pre-cooled sterile tube. Add loading buffer and mix well. Boil at 100 °C for 10 min. Add the prepared sample to the sample well and perform electrophoresis, membrane transfer, blocking, antibody incubation and development in sequence.
2.6. ELISA trial
After adding the sample to the sample well, incubate in a 37 °C incubator for 1 h, then add a chromogenic agent to develop the colour and measure the absorbance (OD value) of each well sequentially. Calculate the concentration of pepsin and trypsin in the sample according to the linear regression equation of the standard curve.
2.7. Acid-suppressive treatment (AST)
Patients who tested pepsin or trypsin positive (57 children and 50 adults) were randomly divided into two groups, one receiving AST (proton pump inhibitors for 8 weeks) as the AST group and the other being followed as the non-AST group. We prepared 29 AST cards and 28 non-AST cards for the Child group, mixed and put them in the Child Group Draw Box. For the Adult group, 25 AST cards and 25 non-AST cards were prepared, shuffled and placed in the Adult Group Draw Box. Subjects decided whether they were in the AST group or the non-AST group by drawing cards from the draw boxes and children had their parents do the drawing for them.
Subjects in both the AST and non-AST groups are required to undergo monthly follow-up examinations, including endoscopic photography of the tympanic membrane, pure tone audiometry (adult)/ABR (child) and acoustic immittance measurement. Tympanic membrane perforation, middle ear effusion, tinnitus and hearing loss related conditions were observed and recorded. After 1 year, the recurrence rate of OME was calculated and the observation was terminated.
2.8. Statistical analysis
Statistical analysis was performed using SPSS version 25.0. Quantitative data in this study were all assessed using the Shapiro-Wilk test, and data with normal distribution were expressed as mean [SD]. Data with non-normal distribution were represented by median and 25 %, 75 % interquartile range (QL, QU). If the data met both normal distribution and homogeneity of variance, the independent samples t-test was used for comparison between samples; otherwise, the Mann-Whitney test was used for comparison between samples. The Chi-square test was used to compare rates. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001).
3. Results and discussion
3.1. Study cohort demographic characteristics
The study involved a total of 320 volunteers, including 270 COME patients and 50 healthy volunteers. The 270 COME patients included 120 children (range, 1–17 years; median age, 6 years) and 150 adults (range, 18–70 years; median age, 43 years). Each group is further divided into serous and mucous groups based on the characteristics of MEE, and the age and gender distribution are shown in Table 1. There was no statistically significant difference in age or gender (P > 0.05). 50 healthy volunteers (range, 18–50 years; mean [SD] age, 34.14 [9.003] years) served as the control group, with a male-to-female ratio of 1:1.
Table 1.
The demographic characteristics of the study populations (COME patients).
| Groups | Variables | Serous (n = 134) | Mucous (n = 136) | t/z-value | p-value |
|---|---|---|---|---|---|
| Child | Age (years) | 6 (4, 11) | 5 (4, 6) | −1.2 | 0.2 |
| Male/Female (n) | 26/30 | 33/31 | 0.3 | 0.6 | |
| Adult | Age | 41 (29, 55) | 44 (31, 55) | −0.4 | 0.7 |
| Male/Female (n) | 37/41 | 35/37 | 0.0 | 0.9 |
3.2. Comparison of RSI positivity rate and WB positivity rate
In this cohort study of 270 COME individuals, the prevalence of trypsin and pepsin in MEE was 39.63 % (107/270). The WB-positive rates of pepsin and trypsin in children are significantly higher than those in adults, suggesting that the incidence rate of LPR in COME children is higher than in adults. However, there was no significant difference in the RSI positivity rate between children and adults, which may be due to the inaccurate expression of reflux symptoms in children. In addition, research has demonstrated that some patients with OME combined with LPR do not have reflux symptoms [20]. The RSI and WB test results in the control group were both negative (see Table 2).
Table 2.
Comparison of RSI positivity rate and WB positivity rate.
| Variables | Child (n = 120) | Adult (n = 150) | x2-value | p-value |
|---|---|---|---|---|
| RSI positivity rate | 25 % | 32.7 % | 1.9 | 0.2 |
| WB positivity rate of pepsin | 47.5 % | 33.3 % | 5.6 | ∗ |
| WB positivity rate of trypsin | 41.7 % | 23.3 % | 10.4 | ∗∗ |
3.3. WB results of trypsin and pepsin in the MEE
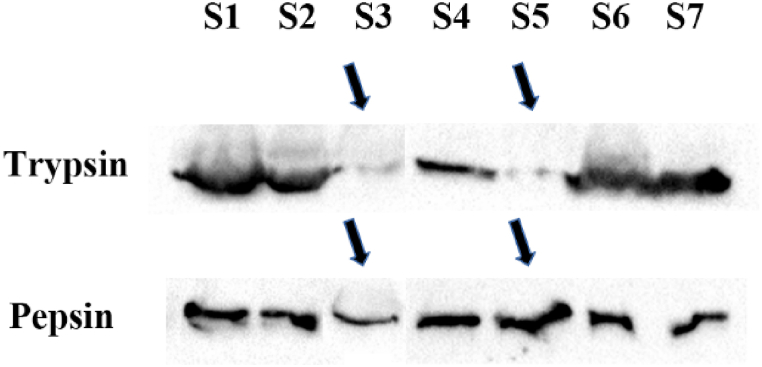
Interestingly, as shown in Fig. 2, in both the child and adult groups, pepsin was positive in trypsin-positive samples. However, trypsin was only detected in some of the pepsin-positive samples. Considering that the anatomical location of pancreatic juice is lower than that of gastric juice, the possibility of trypsin reflux into the nasopharynx during reflux events is less than that of pepsin. Given this, after collecting MEE from OME patients, only pepsin testing could be sufficient to assess whether they have LPR.
Fig. 2.
Representative WB images of trypsin and pepsin in the MEE of 7 COME volunteers.
3.4. ELISA results of trypsin and pepsin in the MEE
Samples that were trypsin or pepsin positive by WB were subjected to further ELISA testing. The concentrations of pepsin and trypsin in MEE were 83.1 (71.3, 89.5) ng/mL and 401.2 (369.8, 462.5) ng/mL in the child group and 55.2 [19.3] ng/mL and 304.6 (289.5, 397.5) ng/mL in the adult group. As shown in Table 3, the type of MEE, serous or mucous, is not significantly associated with the concentration of trypsin or pepsin. Furthermore, the concentration of trypsin and pepsin was significantly higher than that of serum, which also confirmed that the trypsin and pepsin in the MEE did not originate from serum.
Table 3.
Analysis of ELISA results between serous and mucous groups.
| Groups | Variables | Serous (n = 58) | Mucous (n = 49) | t/z -value | p-value |
|---|---|---|---|---|---|
| Child | Pepsin (ng/mL) | 83.7 (63.5, 89.4) | 83.1 (76.5, 90.4) | −0.5 | 0.6 |
| Trypsin (ng/mL) | 398.1 (370.9, 421.2) | 428.2 (362.8, 483.4) | −1.1 | 0.3 | |
| Adult | Pepsin (ng/mL) | 54.6 [19.9] | 55.8 [19.1] | −0.2 | 0.8 |
| Trypsin (ng/mL) | 304.6 (256.4, 394.4) | 318.2 (295.5, 412.3) | −0.6 | 0.5 |
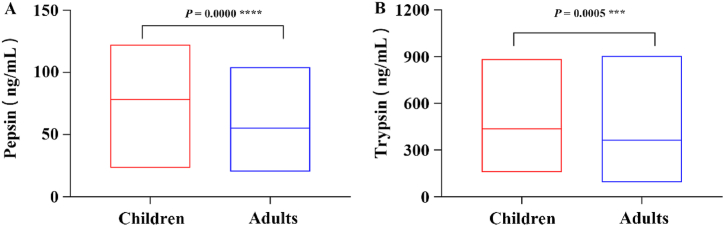
As shown in Fig. 3 and Table 4, both pepsin and trypsin concentrations were higher in children than in adults (P < 0.05), suggesting that LPR may be more severe in children with COME than in adults. In children, the Eustachian tube is almost horizontal, short, about half the length of the adult Eustachian tube, but has a large internal diameter [21]. Children with weak gastrointestinal function and a preference for sweets and fizzy drinks can increase the incidence of LPR. Young children also have poor verbal expression. All of this ultimately leads to a higher incidence and severity of LPR in children with SOM compared to adults.
Fig. 3.
Comparison of the concentrations of (A) pepsin and (B) trypsin in MEE between child and adult group by ELISA.
Table 4.
Comparison of ELISA results and pH between children and adults.
| Groups | Variables | Child (n = 57) | Adult (n = 50) | z/t-value | p-value |
|---|---|---|---|---|---|
| Serous | Pepsin | 83.7 (63.5, 89.4) | 54.6 [19.9] | −3.5 | ∗∗∗ |
| Trypsin | 398.1 (370.9, 421.2) | 304.6 (256.4, 394.4) | −2.6 | ∗∗ | |
| Mucous | Pepsin | 83.1 (76.5, 90.4) | 55.8 [19.1] | −3.8 | ∗∗∗ |
| Trypsin | 428.2 (362.8, 483.4) | 318.2 (295.5, 412.3) | −2.3 | ∗ | |
| pH-value | 7.4 (7.1, 7.6) | 7.4 (7.1, 7.7) | −0.0 | 1.0 | |
Considering that the optimal pH range for pepsin is 1.8–3.5, and pepsin is only active in acidic environments, whereas the optimal pH range for trypsin is between 7.8 and 8.5, and trypsin hardly loses activity in highly acidic environments. The middle ear cavity is a neutral environment (as shown in Table 4) that is more conducive to trypsin function, which has a stronger digestive action to exert destructive effects. In the middle ear environment, activated trypsin can further activate trypsinogen to convert to trypsin, and pepsin is in an inactivated state. The specific pathogenesis requires further research and investigation.
3.5. Evaluation of the effect of 8 weeks-AST on COME
Patients who tested pepsin or trypsin positive were randomly divided into two groups, one receiving AST (proton pump inhibitors for 8 weeks) and the other being followed. 1 year later, the recurrence rate of OME was calculated. As shown in Table 5, in both the child and adult groups, the recurrence rate of OME in the AST group is significantly lower than in the non-AST group.
Table 5.
The recurrence rate of OME within 1 year.
| Groups | Variables | Non-recurrence rate (n = 62) | Recurrence rate (n = 45) | X2-value | p-value |
|---|---|---|---|---|---|
| Child | AST | 86.2 % | 13.8 % | 19.4 | ∗∗∗∗ |
| Non-AST | 28.6 % | 71.4 % | |||
| Adult | AST | 92 % | 8 % | 23.7 | ∗∗∗∗ |
| Non-AST | 24 % | 76 % |
We also recorded the healing time of the tympanic membrane and other complications of tympanic membrane puncture, including tympanic membrane perforation, suppurative otitis media, hearing loss and tinnitus (see Table 6). The results showed that the healing time of the tympanic membrane was significantly shorter in the AST group than in the non-AST group, whereas there was no significant difference in the incidence of tympanic membrane perforation between the two groups. Most tympanic puncture sites are completely healed at the first follow-up visit after one month, which may be due to strict disinfection of the external acoustic meatus before puncture and advice to patients to avoid water entering the external acoustic meatus after puncture. In addition, the incidence of suppurative otitis media, hearing loss and tinnitus was significantly lower in the AST group than in the non-AST group, indicating a significant therapeutic effect of AST on COME.
Table 6.
Evaluation of the effect of 8 weeks-AST on COME.
| Groups | AST (n = 54) | Non-AST (n = 53) | Z/X2-value | p-value |
|---|---|---|---|---|
| a | 1.1 month | 1.6 month | −3.9 | ∗∗∗ |
| b | 3.7 % | 15.1 % | 2.9 | 0.1 |
| Non-b | 96.3 % | 84.9 % | ||
| c | 1.9 % | 18.9 % | 8.4 | ∗∗ |
| Non-c | 98.1 % | 81.1 % | ||
| d | 5.6 % | 24.5 % | 7.6 | ∗∗ |
| Non-d | 94.4 % | 75.5 % | ||
| e | 9.3 % | 28.3 % | 6.4 | ∗ |
| Non-e | 90.7 % | 71.7 % |
∗a, average healing time of the tympanic membrane; b, tympanic membrane perforation; c, suppurative otitis media; d, hearing loss; e, tinnitus.
4. Conclusion
In clinical practice, patients with COME could be provided pepsin detection of MEE to determine if they have LPR. In patients with OME combined with LPR, early acid-suppressive treatment can reduce the recurrence of OME.
CRediT authorship contribution statement
Zeqi Zhao: Writing – original draft, Project administration, Methodology, Data curation, Conceptualization. Zhengzhong Han: Writing – original draft, Methodology, Conceptualization. Yudi Shao: Validation, Methodology, Data curation. Tingting Tang: Methodology, Data curation. Caiji Wang: Resources. Bing Xu: Investigation. Zhenlu Zhao: Resources, Data curation. Xi Shi: Validation. Dan Jin: Data curation. Wei Li: Writing – review & editing, Conceptualization.
Ethics and consent
All experiments were conducted in accordance with protocols approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (ethics number: XYFY2018-KL501-01, ethics approval date: January 2, 2018).
All adult subjects signed a written informed consent form. All minors provided their own verbal informed consent, as well as a written informed consent form from their parents/guardians.
We confirm that, in addition to parental/guardian consent, minor(s) consent was obtained for all experiments.
This study followed the STROBE guidelines.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Basic Research Program of Xuzhou Department of Science and Technology (KC23039), Project supported by the Affiliated Hospital of Xuzhou Medical University, China (2021ZA44), Basic Research Program of Xuzhou Department of Science and Technology (KC21050), Medical research project of Jiangsu Commission of Health, China (H2023032), Social Development Project of Xuzhou Department of Science, Technology (KC22104), Development aid Project of Xuzhou Science and Technology Bureau, China (KC21249).
Contributor Information
Zeqi Zhao, Email: zqzhao@xzhmu.edu.cn.
Zhengzhong Han, Email: zzhan@xzhmu.edu.cn.
Yudi Shao, Email: syd2998@163.com.
Tingting Tang, Email: tangtingt0329@163.com.
Caiji Wang, Email: caiji1983@126.com.
Bing Xu, Email: 15162168020@163.com.
Zhenlu Zhao, Email: 1074798996@qq.com.
Xi Shi, Email: shixi19850212@126.com.
Dan Jin, Email: jindan2789@163.com.
Wei Li, Email: lili78163@163.com.
References
- 1.Pershad A.R., Knox E.C., Shah R.K., Zalzal H.G. Disparities in the prevalence and management of otitis media: a systematic review. Int. J. Pediatr. Otorhinolaryngol. 2024;176 doi: 10.1016/j.ijporl.2023.111786. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld R.M., Shin J.J., Schwartz S.R., Coggins R., Gagnon L., Hackell J.M., Hoelting D., Hunter L.L., Kummer A.W., Payne S.C., Poe D.S., Veling M., Vila P.M., Walsh S.A., Corrigan M.D. Clinical practice guideline: otitis media with effusion executive summary (update) Otolaryngol. Head Neck Surg. 2016;154:201–214. doi: 10.1177/0194599815624407. [DOI] [PubMed] [Google Scholar]
- 3.Mulvaney C.A., Galbraith K., Webster K.E., Rana M., Connolly R., Tudor-Green B., Marom T., Daniel M., Venekamp R.P., Schilder A.G., MacKeith S. Topical and oral steroids for otitis media with effusion (OME) in children. Cochrane Database Syst. Rev. 2023;12 doi: 10.1002/14651858.CD015255.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanai R., Kaneko K. Negative middle ear pressure and otitis media with effusion after surgery under general anesthesia. Acta Otolaryngol. 2012;132:1049–1053. doi: 10.3109/00016489.2012.687455. [DOI] [PubMed] [Google Scholar]
- 5.Fan W., Xu H., Chen F., Li X. The expression of Nrf2 and TLRs in ear effusion in children with different types of otitis media and their relationship with inflammatory factors. Int. Immunopharm. 2024;126 doi: 10.1016/j.intimp.2023.111152. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer E.A., Joseph M., Dunn J., Weiner H.L., Dimachkieh A., Flores A.R., Sanson M.A., Ayele H., Hanson B.M., Kaplan S.L., Vallejo J.G., McNeil J.C. Increasing incidence of Streptococcus anginosus group intracranial infections associated with sinusitis, otitis media, and mastoiditis in children. Pediatr. Infect. Dis. J. 2024;43:e261–e267. doi: 10.1097/INF.0000000000004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juszczak H.M., Loftus P.A. Role of allergy in eustachian tube dysfunction. Curr. Allergy Asthma Rep. 2020;20:54. doi: 10.1007/s11882-020-00951-3. [DOI] [PubMed] [Google Scholar]
- 8.Karyanta M., Satrowiyoto S., Wulandari D.P. Prevalence ratio of otitis media with effusion in laryngopharyngeal reflux. Int J Otolaryngol. 2019;2019 doi: 10.1155/2019/7460891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin X., Liu L., Luo M., Liu Y., Duan M. Association between secretory otitis media and laryngopharygeal reflux in adults. Acta Otolaryngol. 2023;143:946–950. doi: 10.1080/00016489.2024.2302317. [DOI] [PubMed] [Google Scholar]
- 10.Dogru M., Kuran G., Haytoglu S., Dengiz R., Arikan O.K. Role of laryngopharyngeal reflux in the pathogenesis of otitis media with effusion. J Int Adv Otol. 2015;11:66–71. doi: 10.5152/iao.2015.642. [DOI] [PubMed] [Google Scholar]
- 11.Formanek M., Zelenik K., Kominek P., Matousek P. Diagnosis of extraesophageal reflux in children with chronic otitis media with effusion using Peptest. Int. J. Pediatr. Otorhinolaryngol. 2015;79:677–679. doi: 10.1016/j.ijporl.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Krause A.J., Yadlapati R. Review article: diagnosis and management of laryngopharyngeal reflux, Aliment. Pharmacol. Ther. 2024;59(5):616–631. doi: 10.1111/apt.17858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D., Qian T., Sun S., Jiang J.J. Laryngopharyngeal reflux and inflammatory responses in mucosal barrier dysfunction of the upper aerodigestive tract. J. Inflamm. Res. 2020;13:1291–1304. doi: 10.2147/JIR.S282809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zenzeri L., Quitadamo P., Tambucci R., Ummarino D., Poziello A., Miele E., Staiano A. Role of non-acid gastro-esophageal reflux in children with respiratory symptoms. Pediatr. Pulmonol. 2017;52:669–674. doi: 10.1002/ppul.23619. [DOI] [PubMed] [Google Scholar]
- 15.Horvath L., Hagmann P., Burri E., Kraft M. Evaluation of oropharyngeal pH-monitoring in the assessment of laryngopharyngeal reflux. J. Clin. Med. 2021;10 doi: 10.3390/jcm10112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han H., Zhao Y., Lv Q., Liu J., Liang Y. J Voice; 2023. Reliability and Validity of the Chinese Version of Reflux Symptom Score. [DOI] [PubMed] [Google Scholar]
- 17.Weitzendorfer M., Antoniou S.A., Schredl P., Witzel K., Weitzendorfer I.C., Majerus A., Emmanuel K., Koch O.O. Pepsin and oropharyngeal pH monitoring to diagnose patients with laryngopharyngeal reflux. Laryngoscope. 2020;130:1780–1786. doi: 10.1002/lary.28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaffenberger T.M., Belsky M.A., Oberlies N.R., Kumar A., Donohue J.P., Yang T.S., Shaffer A.D., Chi D.H. Long-term impact of middle ear effusion in pediatric tympanostomy tubes. Laryngoscope. 2021;131:E993–E997. doi: 10.1002/lary.28860. [DOI] [PubMed] [Google Scholar]
- 19.Rao Y., Zhang Q., Wang X., Xue X., Ma W., Xu L., Xing S. Automated diagnosis of adenoid hypertrophy with lateral cephalogram in children based on multi-scale local attention. Sci. Rep. 2024;14 doi: 10.1038/s41598-024-69827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han H., Lv Q. Characteristics of laryngopharyngeal reflux in patients with chronic otitis media. Am. J. Otolaryngol. 2018;39:493–496. doi: 10.1016/j.amjoto.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Webster K.E., Mulvaney C.A., Galbraith K., Rana M., Marom T., Daniel M., Venekamp R.P., Schilder A.G., MacKeith S. Autoinflation for otitis media with effusion (OME) in children. Cochrane Database Syst. Rev. 2023;9 doi: 10.1002/14651858.CD015253.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.