Abstract
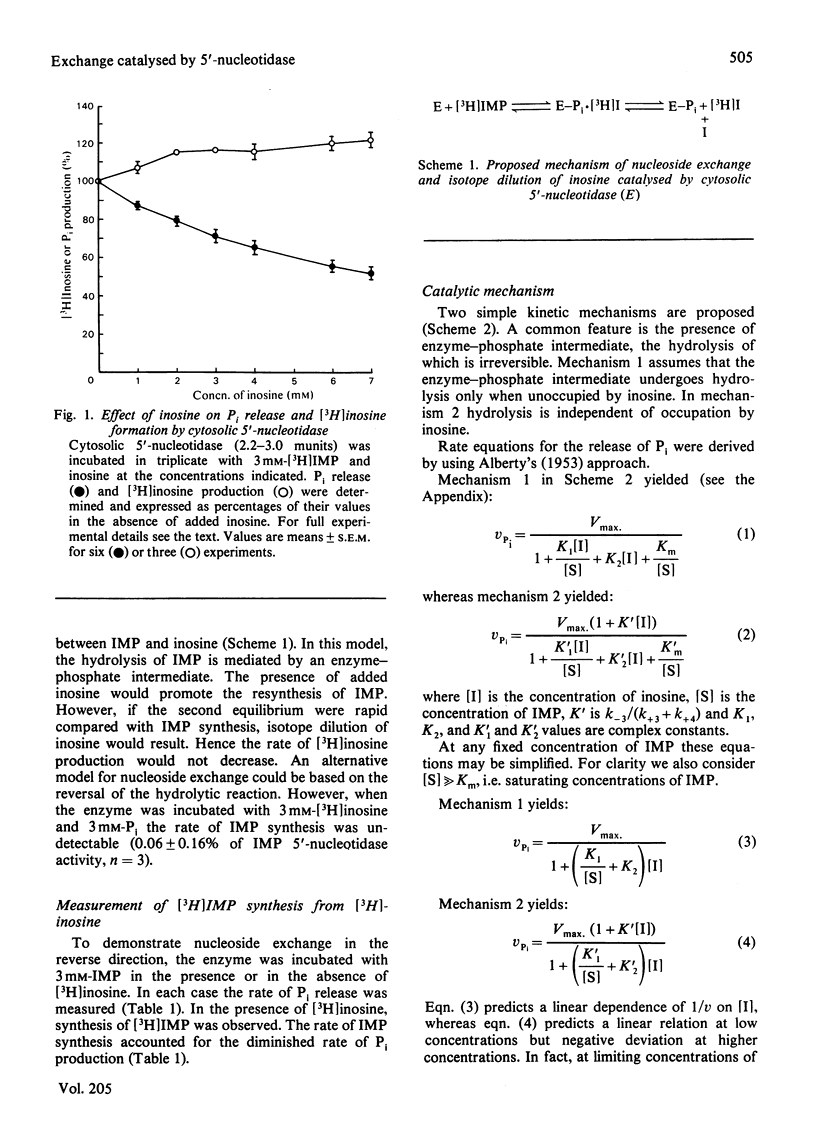
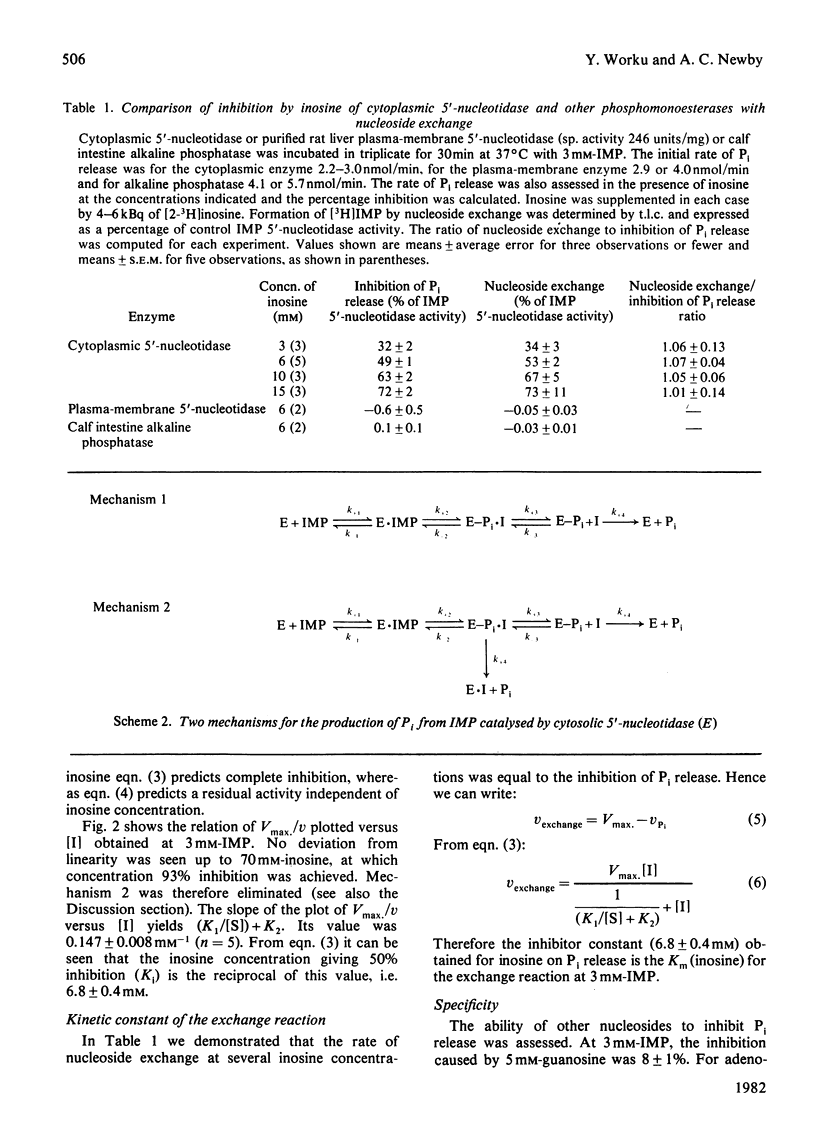
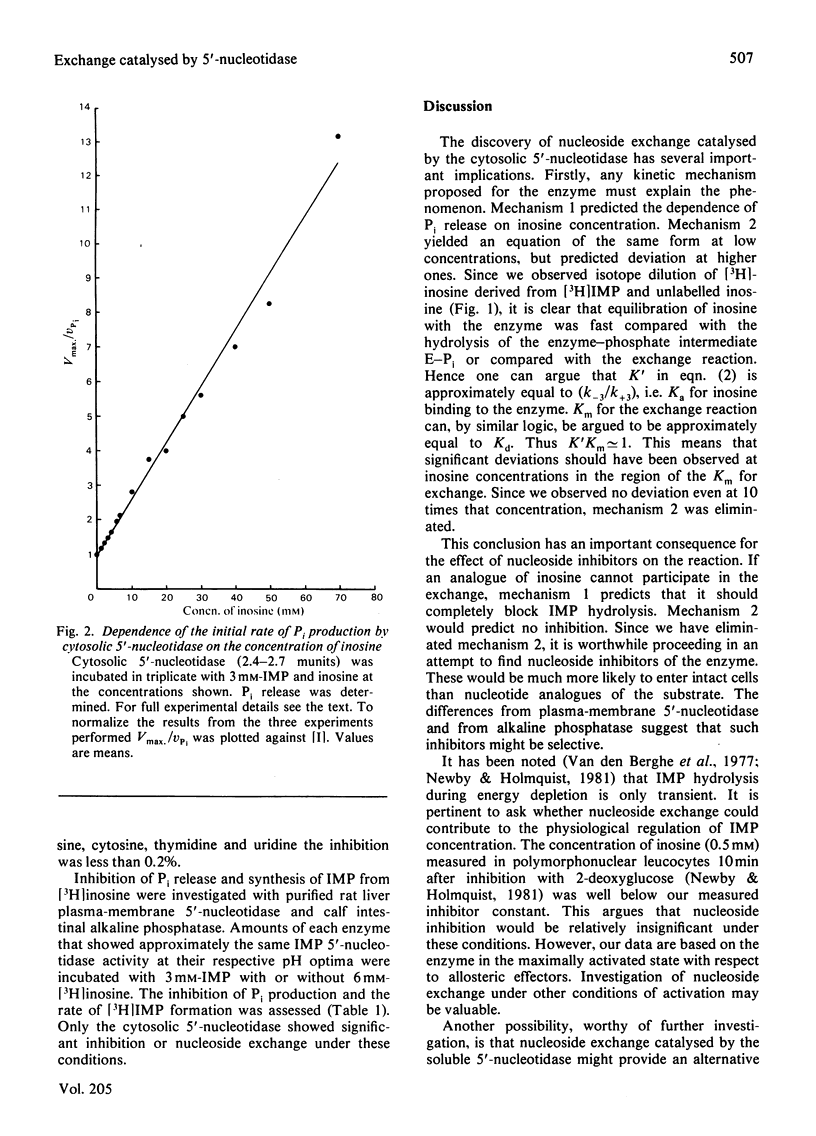
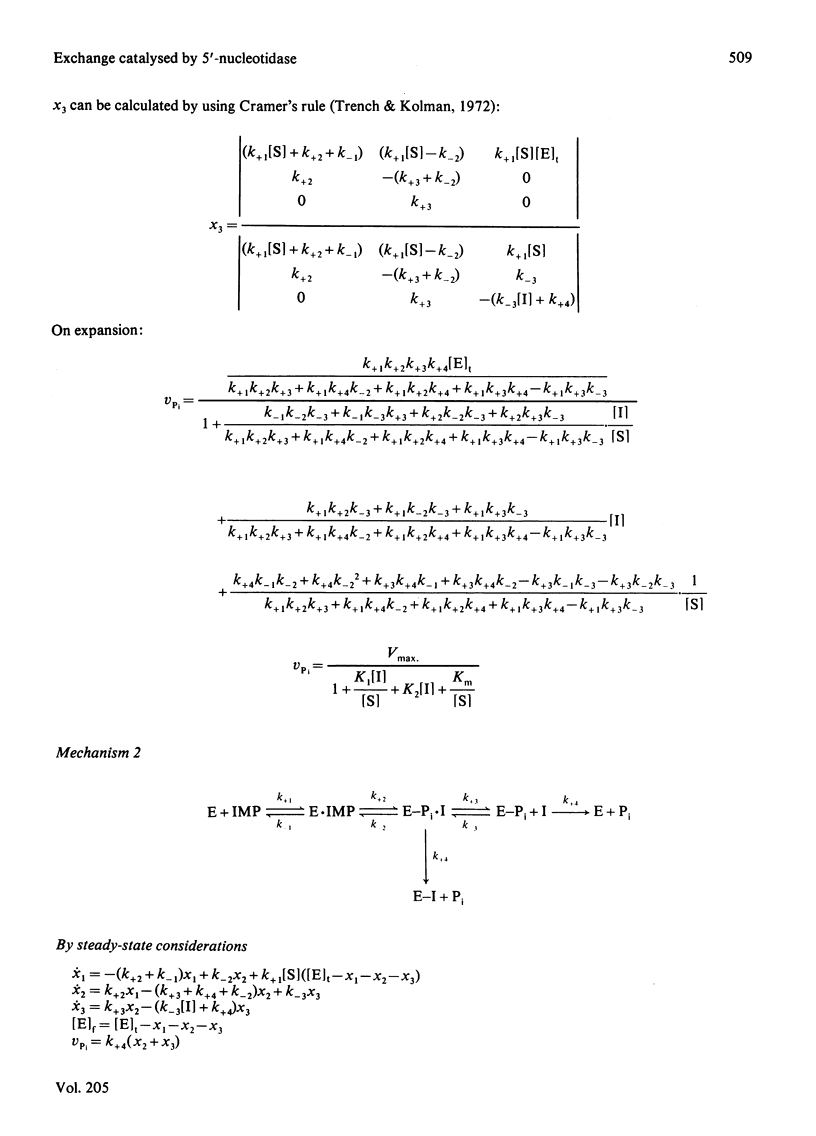
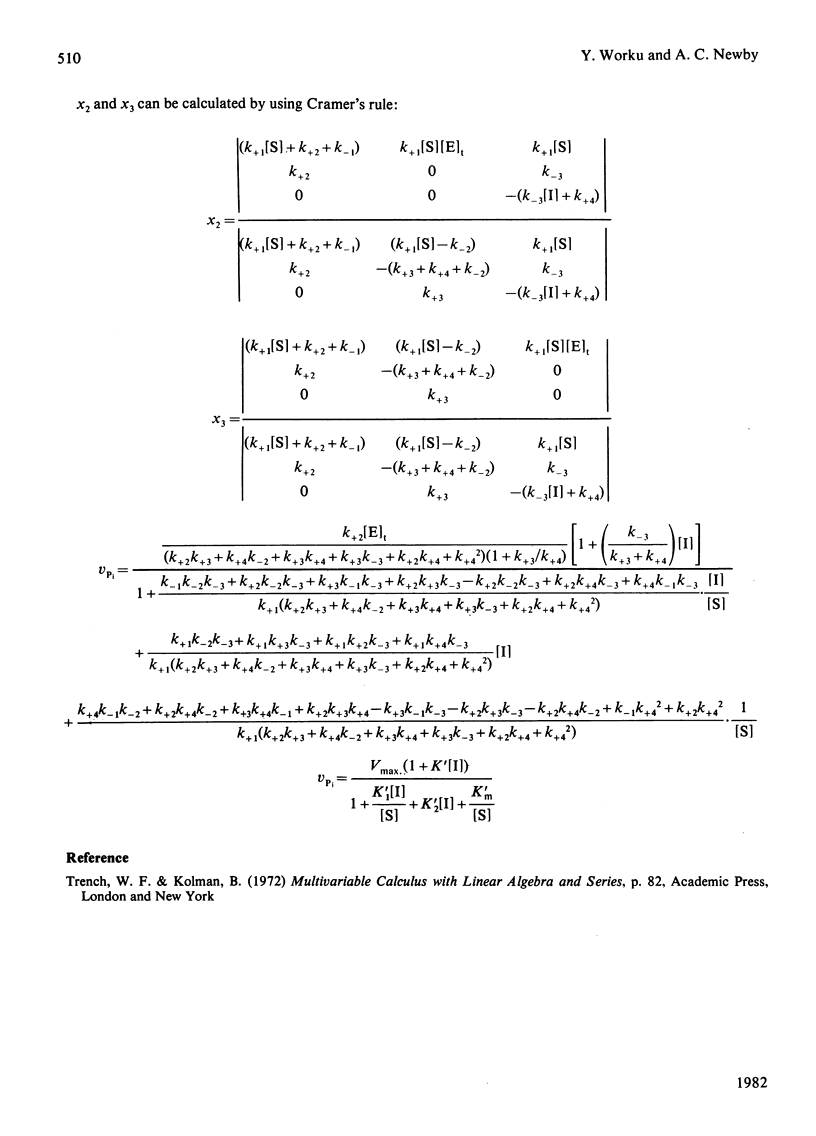
The inhibition of the cytoplasmic 5'-nucleotidase (EC 3.1.3.5) by its product, inosine, was studied with a partially purified preparation of the enzyme from rat liver. Inhibition of Pi production was found to be due to exchange of the inosine moiety between inosine and IMP. Exchange was not catalysed by reversal of the hydrolytic reaction, suggesting, instead, the mediation of an enzyme-phosphate intermediate. Two models for the catalytic mechanism are proposed and rate equations for the dependence of Pi production on inosine concentration are derived. The experimentally determined dependence was consistent with a mechanism in which hydrolysis of the enzyme-phosphate intermediate occurred only when it was unoccupied by inosine. This conclusion suggests that inosine analogues that cannot participate in exchange should inhibit the enzyme. Such inhibitors might be useful in defining the enzyme's physiological role or as pharmacological agents to decrease breakdown of purine nucleotides. The possibility that nucleoside exchange provides an alternative route for the phosphorylation of mutagenic or cytotoxic nucleoside analogues should also be considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Bailyes E. M., Newby A. C., Siddle K., Luzio J. P. Solubilization and purification of rat liver 5'-nucleotidase by use of a zwitterionic detergent and a monoclonal-antibody immunoadsorbent. Biochem J. 1982 Apr 1;203(1):245–251. doi: 10.1042/bj2030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Chapman A. G., Miller A. L., Atkinson D. E. Role of the adenylate deaminase reaction in regulation of adenine nucleotide metabolism in Ehrlich ascites tumor cells. Cancer Res. 1976 Mar;36(3):1144–1150. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Itoh R., Mitsui A., Tsushima K. 5'-nucleotidase of chicken liver. Biochim Biophys Acta. 1967 Sep 12;146(1):151–159. doi: 10.1016/0005-2744(67)90081-2. [DOI] [PubMed] [Google Scholar]
- Itoh R. Purification and some properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Itoh R. Regulation of cytosol 5'-nucleotidase by adenylate energy charge. Biochim Biophys Acta. 1981 May 14;659(1):31–37. doi: 10.1016/0005-2744(81)90268-0. [DOI] [PubMed] [Google Scholar]
- Itoh R., Usami C., Nishino T., Tsushima K. Kinetic properties of cytosol 5'-nucleotidase from chicken liver. Biochim Biophys Acta. 1978 Sep 11;526(1):154–162. doi: 10.1016/0005-2744(78)90300-5. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H., Koszalka G. W., Rideout J. L., Beacham L. M., 3rd, Chao E. Y., Haggerty J. J., Krenitsky T. A., Elion G. B. Adenosine kinase from rabbit liver. II. Substrate and inhibitor specificity. J Biol Chem. 1979 Apr 10;254(7):2346–2352. [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A. Adenosine production inside rat polymorphonuclear leucocytes. Biochem J. 1981 Nov 15;200(2):399–403. doi: 10.1042/bj2000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. Role of adenosine deaminase, ecto-(5'-nucleotidase) and ecto-(non-specific phosphatase) in cyanide-induced adenosine monophosphate catabolism in rat polymorphonuclear leucocytes. Biochem J. 1980 Mar 15;186(3):907–918. doi: 10.1042/bj1860907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. The interaction of inhibitors with adenosine metabolising enzymes in intact isolated cells. Biochem Pharmacol. 1981 Sep 15;30(18):2611–2615. doi: 10.1016/0006-2952(81)90589-x. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Kekomäki M. P., Mäenpä P. H. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem Pharmacol. 1969 Oct;18(10):2615–2624. doi: 10.1016/0006-2952(69)90192-0. [DOI] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Creveling C. R., Daly J. Stimulated formation of adenosine 3',5'-cyclic phosphate in cerebral cortex: synergism between electrical activity and biogenic amines. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1033–1040. doi: 10.1073/pnas.65.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


