Abstract
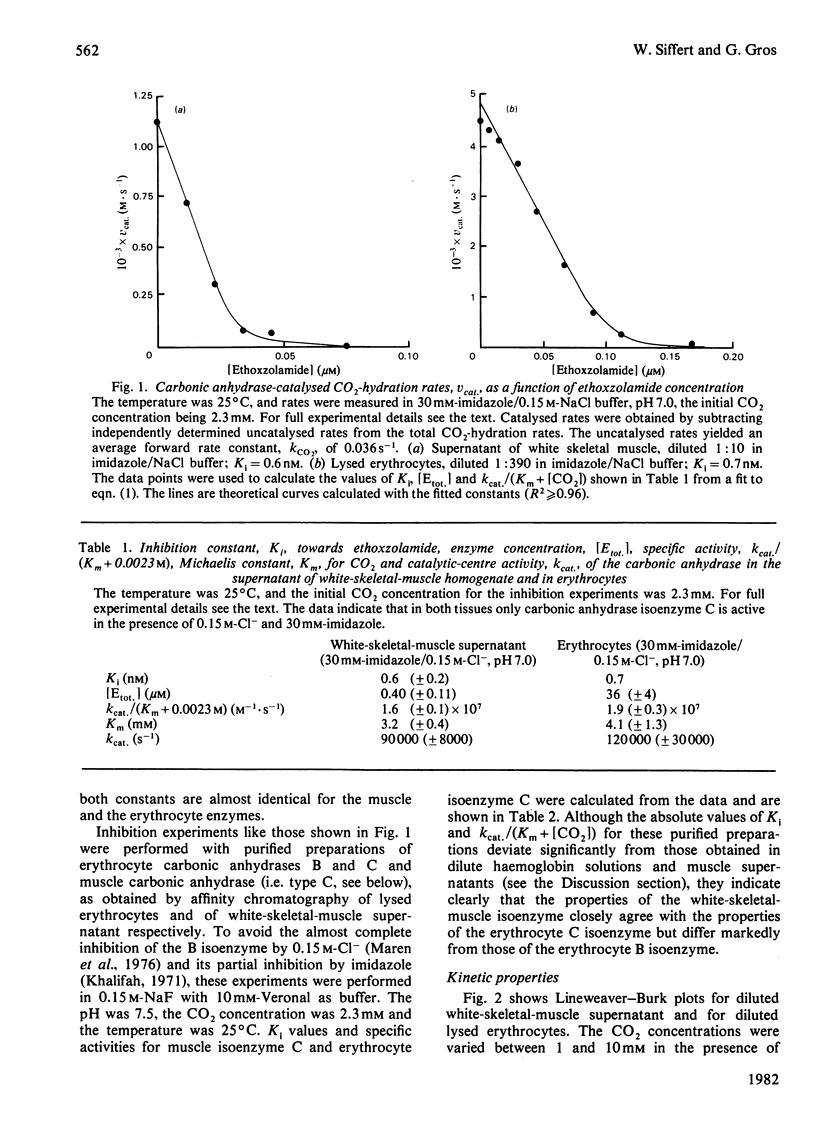
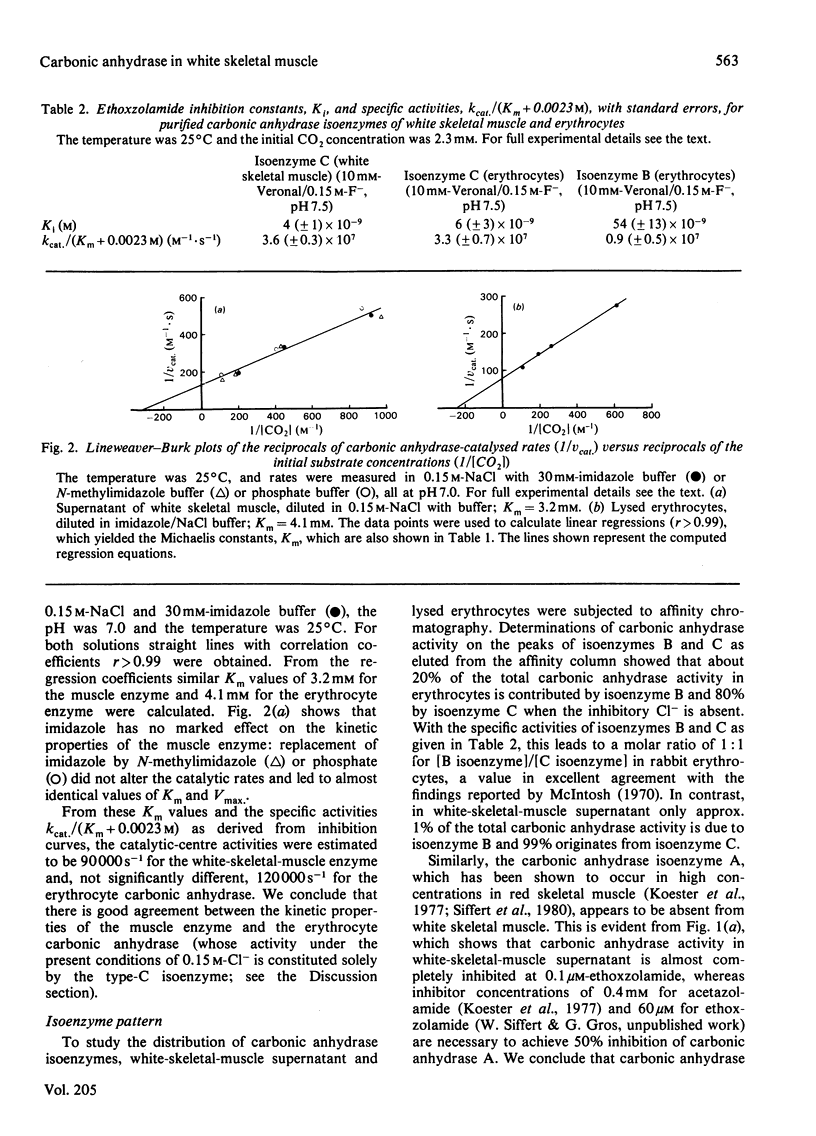
We investigated the activity of carbonic anhydrase in blood-free perfused white skeletal muscles of the rabbit. Carbonic anhydrase activities were measured in supernatants and in Triton extracts of the particulate fractions of white-skeletal-muscle homogenate by using a rapid-reaction stopped-flow apparatus equipped with a pH electrode. An average carbonic anhydrase concentration of about 0.5 microM was determined for white skeletal muscle. This concentration is about 1% of that inside the erythrocyte. Some 85% of the muscle enzyme was found in the homogenate supernatant, and only 15% appeared to be associated with membranes and organelles. White-skeletal-muscle carbonic anhydrase was characterized in terms of its Michaelis constant and catalytic-centre activity (turnover number) for CO2 and its inhibition constant towards ethoxzolamide. These properties were identical with those of the rabbit erythrocyte carbonic anhydrase C, suggesting that a type-C enzyme is present in white skeletal muscle. Affinity chromatography of muscle supernatant and of lysed erythrocytes showed that, whereas rabbit erythrocytes contain about equal amounts of carbonic anhydrase isoenzymes B and C, the B isoenzyme is practically absent from white skeletal muscle. Similarly, ethoxzolamide-inhibition curves suggested that white skeletal muscle contains no carbonic anhydrase A. It is concluded that white skeletal muscle contains essentially one carbonic anhydrase isoenzyme, the C form, most of which is probably of cytosolic origin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter N., Shiels A., Tashian R. Carbonic anhydrase III isoenzyme from human and bovine muscle [proceedings]. Biochem Soc Trans. 1978;6(3):552–553. doi: 10.1042/bst0060552. [DOI] [PubMed] [Google Scholar]
- Crandall E. D., Klocke R. A., Forster R. E. Hydroxyl ion movements across the human erythrocyte membrane. Measurement of rapid pH changes in red cell suspensions. J Gen Physiol. 1971 Jun;57(6):664–683. doi: 10.1085/jgp.57.6.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. M., Weissman M. L. Carbonic anhydrase activity of the cat hind leg. J Appl Physiol Respir Environ Exerc Physiol. 1979 Nov;47(5):1090–1098. doi: 10.1152/jappl.1979.47.5.1090. [DOI] [PubMed] [Google Scholar]
- GRAY S. J., STERLING K. The tagging of red cells and plasma proteins with radioactive chromium. J Clin Invest. 1950 Dec;29(12):1604–1613. doi: 10.1172/JCI102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros G., Forster R. E., Lin L. The carbamate reaction of glycylglycine, plasma, and tissue extracts evaluated by a pH stopped flow apparatus. J Biol Chem. 1976 Jul 25;251(14):4398–4407. [PubMed] [Google Scholar]
- HODGE H. C. Training of toxicologists. Fed Proc. 1960 Sep;19(Suppl 4):50–52. [PubMed] [Google Scholar]
- Kawashiro T., Scheid P. Measurement of Krogh's diffusion constant of CO2 in respiring muscle at various CO2 levels: evidence for facilitated diffusion. Pflugers Arch. 1976 Mar 30;362(2):127–133. doi: 10.1007/BF00583638. [DOI] [PubMed] [Google Scholar]
- Kernohan J. C. A method for studying the kinetics of the inhibition of carbonic anhydrase by sulphonamides. Biochim Biophys Acta. 1966 May 5;118(2):405–412. doi: 10.1016/s0926-6593(66)80049-8. [DOI] [PubMed] [Google Scholar]
- Koester M. K., Register A. M., Noltmann E. A. Basic muscle protein, a third genetic locus isoenzyme of carbonic anhydrase? Biochem Biophys Res Commun. 1977 May 9;76(1):196–204. doi: 10.1016/0006-291x(77)91686-2. [DOI] [PubMed] [Google Scholar]
- MAREN T. H., PARCELL A. L., MALIK M. N. A kinetic analysis of carbonic anhydrase inhibition. J Pharmacol Exp Ther. 1960 Dec;130:389–400. [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Rayburn C. S., Liddell N. E. Inhibition by anions of human red cell carbonic anhydrase B: physiological and biochemical implications. Science. 1976 Feb 6;191(4226):469–472. doi: 10.1126/science.813299. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Wiley C. E. The in vitro activity of sulfonamides against red cell carbonic anhydrases. Effect of ionic and substrate variation on the hydration reaction. J Med Chem. 1968 Mar;11(2):228–232. doi: 10.1021/jm00308a008. [DOI] [PubMed] [Google Scholar]
- McIntosh J. E. Carbonic anhydrase isoenzymes in the erythrocytes and uterus of the rabbit. Biochem J. 1970 Nov;120(2):299–310. doi: 10.1042/bj1200299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muther T. F. On the lack of specificity of the cobalt-bicarbonate method for carbonic anhydrase. J Histochem Cytochem. 1977 Sep;25(9):1043–1050. doi: 10.1177/25.9.71324. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y. Observations on the localization of carbonic anhydrase in muscle. Acta Physiol Scand. 1979 Jun;106(2):239–240. doi: 10.1111/j.1748-1716.1979.tb06393.x. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Maren T. H. Thermodynamics of carbonic anhydrase catalysis. A comparison between human isoenzymes B and C. J Biol Chem. 1981 Jan 25;256(2):608–612. [PubMed] [Google Scholar]
- Zborowska-Sluis D. T., L'Abbate A., Klassen G. A. Evidence of carbonic anhydrase activity in skeletal muscle: a role for facilitative carbon dioxide transport. Respir Physiol. 1974 Sep;21(3):341–350. doi: 10.1016/0034-5687(74)90064-4. [DOI] [PubMed] [Google Scholar]


