Abstract
Comprehensive biomarker testing for patients with non–small cell lung cancer is critical for selecting appropriate targeted therapy or immunotherapy. Ensuring timely ordering, processing, and reporting is key to optimizing patient outcomes. However, various factors can prevent or delay patients from being offered the option of treatment selection based on comprehensive biomarker testing. These factors include problems with access to testing, tissue adequacy, turnaround time, and health insurance coverage and billing practices. Turnaround time depends on several logistical and tissue handling factors, which involve institutional policies, processes, resources, testing methodology, and testing algorithms that vary across different practices. In this article, the authors identify key factors that prolong biomarker testing turnaround time, propose strategies to reduce it, and present a process map to aid physicians and key organizational stakeholders in improving testing efficiency.
Keywords: comprehensive biomarker testing, non–small cell lung cancer, process map, quality assessment, quality improvement, quality metrics, testing algorithms, testing efficiency, tissue adequacy, turnaround time
Short abstract
Ensuring timely ordering, processing, and reporting of comprehensive biomarker testing for patients with non–small cell lung cancer is key to optimizing patient outcomes. The authors identify key factors that prolong biomarker testing turnaround time, propose strategies to reduce it, and present a process map to aid physicians and key organizational stakeholders in improving testing efficiency.
INTRODUCTION
Optimal treatment options for patients with non–small cell lung cancer (NSCLC) require comprehensive biomarker testing to guide clinical decision making. 1 Clinical practice guidelines from professional societies, including the National Comprehensive Cancer Network, the College of American Pathologists (CAP)/International Association for the Study of Lung Cancer (IASLC)/Association of Molecular Pathologists (AMP), the American Society of Clinical Oncology, and the European Society for Medical Oncology, all recommend biomarker testing for patients with newly diagnosed NSCLC. 1 , 2 , 3 , 4 , 5
In addition to identifying targetable genomic biomarkers, evaluating programmed death‐ligand 1 (PD‐L1) expression by immunohistochemistry (IHC) can identify patients who may benefit from immune checkpoint inhibition as initial monotherapy or in the adjuvant setting. 6 , 7 , 8 , 9 , 10 Therefore, obtaining both genomic and immunohistochemical biomarkers is essential to determine the optimal treatment for patients with NSCLC. Further highlighting the need for comprehensive biomarker testing, patients with EGFR, ALK, and ROS1 alterations are unlikely to respond to frontline immunotherapies. 11 Administration of immunotherapy before some tyrosine kinase inhibitor therapies exposes patients to increased and avoidable toxicity. 8 , 12 , 13
Despite the growing evidence of clinical benefit from biomarker‐driven treatment selection, many patients with metastatic NSCLC do not receive testing for biomarkers as recommended by existing guidelines. 14 , 15 , 16 , 17 , 18 A survey by the IASLC reported higher testing rates for biomarkers with the longest standing approvals for targeted therapies, such as EGFR and ALK (ranging between 83% and 94%); however, biomarkers like ROS1, BRAF, KRAS, MET, RET, and ERBB2 (HER2), which guide the selection of specific targeted therapies that were approved after 2013, had much lower rates of testing (ranging from 20% to 69%). 19 With the increasing number of biomarkers needed to guide therapeutic decisions for patients who have NSCLC, sequential testing strategies for single gene abnormalities now run the risk of depleting available tissue and increasing the turnaround time (TAT) from initial test requisition to availability of actionable results. 14 , 15 , 20 Therefore, a strategy of timely comprehensive biomarker testing, in which all biomarkers that test eligibility for approved therapies are performed simultaneously, is increasingly advocated. 21 , 22 We support the practice of comprehensive biomarker testing, whenever possible, to identify optimal treatments for patients. It is worth clarifying because there is variation in actual practice for assays used and the comprehensiveness of biomarkers assessed across different institutions. Over time, the term biomarker testing throughout this statement refers to assessing any treatment‐predictive biomarkers using available assays.
In addition to the importance of comprehensive biomarker testing and addressing other factors that delay the time from diagnosis to treatment, the TAT of biomarker results is needed to capitalize on the improved outcomes offered by effective targeted and immune therapies. Evidence from single centers shows that TAT varies and may span from within the CAP/IASLC/AMP recommended 10 working days to well over 1 month. 17 , 23 , 24 , 25 , 26 , 27 Evidence also suggests that delays in TAT are a barrier to optimal care. 14 , 20 , 24 One study indicated that approximately 20% of patients with metastatic NSCLC undergo chemotherapy before genotyping results for EGFR or ALK are available. 20 Another nationwide biomarker survey in France demonstrated that long TATs caused local tumor boards to recommend conventional chemotherapy rather than waiting for biomarker test results. 24 To better define the factors that influence TAT, it is essential to recognize the series of interconnected steps occurring across multiple disciplines, each of which may contribute to lengthy TAT through poor coordination, inefficiencies, and lack of communication. This article focuses on the key issues related to prolonged TAT, from tissue delivery to the pathology department to reporting biomarker test results.
The tissue journey: From diagnosis to biomarker testing
A primary care clinician (physician or nurse practitioner) or specialist (commonly pulmonologist and thoracic surgeon) frequently initiates the initial evaluation of suspected NSCLC after symptoms, a radiologic abnormality found incidentally, or by low‐dose computed tomography screening, which leads to tissue procurement by an interventional radiologist, pulmonologist, or thoracic surgeon. The procured tissue is then evaluated by a pathologist who confirms the diagnosis of lung cancer and subtypes the tumor (e.g., small cell lung cancer, NSCLC, etc.). For patients who have biomarker testing performed, testing is usually initiated at some point after a diagnosis of NSCLC is made. Some health care physicians participating in the diagnostic process may receive pathology results and order biomarker testing. However, this process is not initiated in most instances until the patient sees a medical oncologist, which may be several days to weeks after the initial diagnosis. In many cases, patients are not offered biomarker testing and proceed immediately to conventional chemotherapy; other patients offered biomarker testing might reject it to avoid treatment delay or unreimbursed expenses; finally, some patients may not receive comprehensive biomarker testing, and their cancer may harbor an actionable mutation that is not assessed. 28 Each instance is a missed opportunity to offer the patient a more informed and potentially superior treatment option.
When biomarker testing is ordered, a pathologist evaluates whether there is adequate tissue. In cases where the tissue is deemed adequate, different laboratory processes are required before it is either directed to in‐house biomarker assays or sent to a commercial or reference laboratory for testing. However, if the available tissue is deemed inadequate, physicians and patients must consider the impact on treatment planning and the risks associated with a repeat biopsy to complete biomarker testing. They may consider plasma‐based testing as an alternative.
Once biomarker testing is initiated, PD‐L1 IHC results are usually available within days (approximately 2–5 days); however, molecular test results for the remaining biomarkers may become available individually over several days (for single‐gene assays) or weeks (for comprehensive, panel‐based molecular profiling). The CAP/IASLC/AMP guideline for tissue‐based molecular testing recommends that, once a diagnosis of NSCLC is rendered, tissue should be sent to the testing laboratory within 3 working days of receiving test requests for reference laboratories and within 24 hours for in‐house laboratories, with a TAT of 10 working days or less for test results to be available (from sample receipt in the molecular laboratory to reporting of all results). 2 , 29
The challenge of timely and comprehensive biomarker testing
Timely ordering, completion, and reporting of comprehensive biomarker testing results have important implications for patient care. Several studies demonstrate that prolonged TAT (≥2 weeks) for biomarker test results can adversely influence clinical decision making; physicians may choose potentially inferior therapy instead of waiting for biomarker test results. 14 , 20 , 24 The decision to proceed with systemic therapy without biomarker guidance may be influenced by patients' or physicians' anxiety. Still, it may indicate clinical urgency to begin treatment, such as declining performance status. Long TAT of genotyping results may prevent up to 50% of patients with metastatic NSCLC from eligibility for enrollment in clinical trials driven by the biomarker results. 30 , 31 With the expanding list of biomarker‐selected treatments, shorter TAT of comprehensive panel‐based biomarker testing is urgently needed. The National Comprehensive Cancer Network and CAP/IASLC/AMP guidelines recommend panel‐based testing, such as next‐generation sequencing (NGS) assays. 1 , 2 However, there is an urgent need to determine optimal and timely testing strategies for different practice settings.
Barriers to large‐scale adoption of comprehensive biomarker testing include unavailability of expanded panel‐based testing, especially in low‐resource settings; variability in adequate tissue acquisition; variable reimbursement for NGS‐based assays; poor physician awareness of clinically relevant biomarkers; limited physician knowledge or expertise in interpreting results; and, in some cases, physician preference for highly reimbursable infusion therapies. 17 , 18 , 32 , 33 These result in inconsistent use of comprehensive biomarker testing across oncology practices.
This article highlights the important timepoints in the workflow from tissue procurement to biomarker reporting and their potential impact on TAT, which varies between institutions and practices. We describe preanalytical and analytical factors that can adversely affect TAT and recommend mitigating strategies. We also propose a process map for physicians and key organizational stakeholders to support greater efficiency and new strategies to reduce TAT.
Factors affecting TAT
TAT for comprehensive biomarker testing should be measured from the day tumor tissue becomes available (typically, once the pathologic diagnosis is finalized) to the day the treating physician receives the molecular test results. For patients with a high pretest probability of NSCLC, the perceived TAT often starts from when the clinical diagnosis is made, even prior to tissue procurement and a pathology diagnosis. The following key factors affect the TAT of lung cancer biomarker tests (Table 1):
TABLE 1.
Factors affecting turnaround time of biomarker test results and possible strategies to optimize testing.
| Factors affecting TAT | Strategy | Challenges and limitations |
|---|---|---|
| Timeliness of test ordering | Health care physician orders molecular testing at the time of initial clinical diagnosis
|
|
| Pathologist‐directed reflex testing based on stage and histology |
|
|
| Availability and adequacy of tissue for testing |
|
|
| Choice of assay |
|
|
| Alternate or complementary testing |
|
|
| Quality metrics |
|
|
Abbreviations: NSCLC, non–small cell lung carcinoma; TAT, turnaround time.
Preanalytical factors
Timeliness of test ordering
Availability and adequacy of tissue for testing
Analytical factors
Choice of assay
Type of testing laboratory and tissue‐handling processes
PREANALYTICAL FACTORS
Timeliness of test ordering
Streamlining biomarker test requests
Delay in initiating biomarker testing is one of the most important preanalytical variables. A high level of local interdisciplinary communication and coordination is central to consistent and timely test ordering. There is no consensus for a single subspecialty to act as the responsible physician. The optimal strategies may vary based on expertise and logistics at the particular practice or institution. 16 Potential challenges experienced by each subspecialty include the following.
Proceduralists: Many pulmonologists, interventional radiologists, and thoracic surgeons who perform the initial diagnostic biopsy do not initiate testing because they may not be comfortable with the testing requirements (for instance, the stage of the lung cancer, the histology appropriate for biomarker testing, the specific biomarkers to order, the testing algorithm to follow, the kind of specimen preparation required for testing), interpretation of tests, or responding to patient inquiries about subsequent management. 33 , 34 For many nonsurgical proceduralists, there is no ongoing physician–patient relationship, and the ordering of any further testing beyond routine diagnostic pathology may be commonly viewed as the treating physician's responsibility.
Pathologists: Pathology departments often wait for biomarker test requests from other physicians to initiate testing, partly because of the current stage‐limited indication for biomarker testing. 1 Pathologists are often uncertain of the clinical stage, so there has been a reluctance to take on the responsibility of test ordering when testing was mostly restricted to patients with stage IV disease. Waiting for a test order request from another treating physician is also a means of compliance with statutes against self‐referral. 35
Oncologists: It often falls on oncologists to order biomarker testing because the results drive their treatment decisions. However, patients often establish care with an oncologist days to weeks after the initial diagnosis of NSCLC. Waiting for the oncologist to order biomarker testing delays the process of informed treatment decision making. Ideally, biomarker results should be available to help formulate a treatment plan when the patient first meets the oncologist. In addition, some oncologists may be reluctant to order biomarker testing until established care is set up or they meet the patient to ensure appropriate follow‐up of test results.
In addition, patients who self‐refer to a tertiary care cancer center often do not have a complete set of reports that outline biomarker testing performed at the initial diagnosis. Possible approaches to address these problems are summarized below in Table 1.
Ordering within the electronic health record
Ordering through an electronic health record (EHR) can help prevent delays in biomarker testing. A templated biomarker panel order‐set request created using the EHR to be initiated within 1 day of the finalized pathology report can improve efficiency. When the pathology report populates the EHR, an automatic notification can alert the appropriate health care physician to place the order. The order may be as simple as “lung cancer comprehensive biomarker testing,” and appropriate biomarkers can be updated using a dynamic interface based on evolving testing guidelines. Thus the physician can be prompted through the EHR to initiate test orders within 1 day of the finalized pathologic diagnosis.
Although EHR optimization has the potential to improve testing, this approach still relies on various physicians ordering testing promptly. For admitted patients, an EHR prompt or message could be sent to the physician responsible for their care; however, this may be less practical for outpatients. It may be unclear which outpatient clinician (primary care physician, interventional radiologist, pulmonologist, thoracic surgeon, nurse practitioner, or someone else) will be responsible for initiating the test order. However, a triage system compatible with the organization could be established to designate a clinician under appropriate circumstances and then alert the designated clinician to initiate biomarker testing for either inpatients or outpatients. One barrier to timely test ordering for inpatients is the Medicare 14‐day rule. This rule precludes independent billing for biomarker testing within 14 days of hospitalization, thus disincentivizing medical systems from providing timely testing for current or recently hospitalized patients. Paradoxically, these patients are even more likely to need timely treatment and thus efficient TAT. 36
Another drawback is that automated notification within the EHR to trigger the biomarker order may not be feasible when practices use different EHRs or hospital EHRs without a closed loop. In some cases, the anatomic pathology laboratory information management system may communicate in only one direction with the hospital EHR, allowing reports to cross over but not accepting incoming orders. Thus testing may rely on less fail‐safe processes, such as faxed or emailed orders. These factors delay biomarker testing, diminishing access to timely testing and effective targeted therapy.
Reflex biomarker testing
One of the most efficient ways to reduce a delay in ordering is to have reflex testing protocols, in which biomarker testing is initiated automatically based on predetermined criteria and institutional policy. 20 , 25 , 37 Reflex testing will become more feasible as the indications for biomarker testing extend across the clinical stage and may eventually be akin to the American Society of Clinical Oncology/CAP guidelines for breast cancer (i.e., reflex fluorescence in situ hybridization [FISH] testing must be performed when ERBB2 [HER2] IHC results are equivocal). 38 Reflex testing could be based on predetermined institutional qualification criteria initiated when a final pathologic diagnosis has been rendered. Several centers have published their experience implementing a reflex testing strategy and have demonstrated improved testing rates and TATs. 25 , 39 , 40 , 41
The potential impact of a reflex strategy on TAT depends on preintervention ordering delays. One center improved the average TAT (from diagnostic pathology report to biomarker report) from 26 days to 15 days in 1 year. 25 To avoid regulatory or reimbursement problems, institutions and practices should establish consensus processes, including clear expectations and communication (ideally through electronic orders in the her, as discussed in the previous section), to ensure timely and appropriate testing. Given the complex logistics involved in the timely diagnosis, staging, and treatment of lung cancer, interdisciplinary communication and coordination are crucial. Pathologists should work closely with the key subspecialists involved in diagnosis and treatment, including interventional radiologists, pulmonologists, medical oncologists, and thoracic surgeons, to establish the rules, roles, and relationships to streamline their institution's workflow. Such processes should consider and communicate the appropriate stage of NSCLC for reflex testing. Similarly, proceduralists working within institutions that adopt reflexive testing should be made aware of such testing so that they routinely aim to procure sufficient tissue for comprehensive biomarker testing to avoid unnecessary additional procedures. Institutional policies for reflexive biomarker testing should consider the current level of clinical evidence, insurance policies, billing requirements, other contractual relationships, and available resources. These approaches to streamlining biomarker test requests can enable a more efficient workflow for timely testing and reporting.
Availability and adequacy of tissue for testing
A critical component of optimal biomarker testing is procuring an adequate tumor sample. Inadequate (quantity not sufficient) tissue samples lengthen TAT by necessitating a search for more suitable material. Often this means scheduling a repeat biopsy or treatment without information from comprehensive biomarker testing. Adequate samples should be allocated for routine tissue processing during the initial procedure. Although some laboratories may be able to use non‐formalin‐fixed paraffin‐embedded (FFPE) specimens, such as cytology smear slides or liquid‐based cytology preparations, 42 , 43 , 44 , 45 most commercial laboratories require FFPE blocks with adequate tumor tissue to further process the sample. Given the differences in laboratory practices, assays, and strategies, it is crucial for those performing tissue sampling to know what adequate tissue means for their testing laboratory. In addition to the size, quality of tissue, and tumor cellularity needed, the feasibility of biomarker testing also depends on judicious specimen processing to ensure minimal tissue depletion. 46 , 47 , 48 Some strategies to minimize quantity not sufficient results are outlined below and summarized in Table 1.
Rapid on‐site evaluation and handling of cytology samples
Rapid on‐site evaluation (ROSE) at the tissue acquisition increases the likelihood of specimen adequacy. 49 CAP guidelines recommend ROSE for all endobronchial ultrasound‐guided, bronchoscopic, and transthoracic biopsies to ensure collection of an adequate sample for comprehensive biomarker testing when the clinical suspicion of lung cancer is high. 50 ROSE on specimens collected by fine‐needle aspiration and touch‐imprint cytology of core‐biopsy samples can also facilitate preordering reflex biomarker testing within the EHR when diagnosing NSCLC. The primary limitation is having the necessary infrastructure, personnel, and resources to support ROSE and the perceived delays in procedure TAT when ROSE is included. 51
With or without ROSE, cytology samples should be prioritized for FFPE cell‐block preparation rather than split for cytospin or liquid‐based cytology (e.g., ThinPrep) and cell block, especially where biomarker testing is performed from FFPE blocks only. A minimal volume of the aspirate (e.g., a drop of the sample) can be used to prepare a direct smear for adequacy assessment by ROSE. The remaining aspirate and at least three—but preferably five—passes can be directly submitted for preparing the FFPE cell block. 50 This would improve yield for diagnosis and comprehensive biomarker testing and potentially help practices that do not have adequate resources for ROSE on cytology samples. For patients who present with malignant pleural or pericardial effusions, the cytologic fluid may be the only specimen available for diagnosis and comprehensive biomarker testing. In these situations, CAP guidelines recommend prioritizing the FFPE cell‐block preparation in anticipation of biomarker testing. 50 Incorporating ROSE during specimen collection to ensure adequacy can reduce delays resulting from insufficient biopsies for comprehensive biomarker testing. 49 , 52 , 53
Optimized handling of small biopsy samples
One option to conserve scanty biopsy specimens is to submit individual cores from different biopsy passes in separate cassettes for processing as individual FFPE tissue blocks. 54 This allows a tissue block with one core‐biopsy sample to be processed with sections for hematoxylin and eosin staining (the first and last cut sections) for diagnosis and provides intervening unstained slides for diagnostic IHCs. The remaining tissue blocks can be preserved for biomarker studies, with only a superficial section used for hematoxylin and eosin staining to allow an assessment of viable tumor adequacy in each core sample. Although this approach may use additional resources in the histology laboratory, it may ultimately be safer and more cost‐effective by reducing the number of inadequate biopsies and the need for repeat procedures. 54
Minimizing diagnostic immunohistochemistry
IHC for diagnostic markers and subtyping specimens should be informed by the clinical context, following recommendations set forth by the IASLC. 55 For most purposes, when IHC is needed for the subtyping of NSCLC, TTF1 and p40 are considered sufficient in clinical practice if there are no morphologic features of neuroendocrine differentiation. If neuroendocrine differentiation is present or metastasis from other primary sites is suspected, additional IHC markers may be used for a more definitive diagnosis. 55 Minimizing the number of diagnostic IHCs performed can conserve tissue for biomarker testing and prevent delays caused by insufficient tissue.
Preidentifying tumor blocks for testing
Pathologists rendering a NSCLC diagnosis should indicate the suitability and availability of tissue blocks for biomarker testing at the time of diagnosis, ideally within the pathology report itself and, at a minimum, within the pathology laboratory information‐management system. This can prevent delays by avoiding re‐reviewing slides to identify the appropriate block for biomarker testing.
Providing tissue‐qualification criteria
Biomarker testing laboratories can provide tissue‐qualification criteria based on biomarker assay performance characteristics. This empowers the primary diagnostic pathologist to select an appropriate sample for biomarker testing that the testing laboratory will deem adequate. Key elements for the tissue‐qualification criteria would include minimal tissue size (mm2); overall cellularity (especially for cytology specimens); tumor cellularity (percentage of tumor cells in the specimen); qualitative features, including the presence of necrosis and extracellular mucin; and a list of agents that can interfere with molecular testing, such as heavy metal fixatives and harsh acid decalcification. 56 These steps can identify specimens that may be suboptimal for accurate biomarker characterization. Documentation of these elements, either directly within the pathology report or in the laboratory information‐management system, can minimize delays in selecting the best specimen for biomarker studies. Laboratory‐specific workflows that adhere to tissue‐qualification guidelines can ensure a streamlined process, minimize delays, and reduce TAT.
ANALYTICAL FACTORS
Choice of assay
Multiple methods are available for biomarker testing, including IHC (PD‐L1, ALK, ROS1), FISH (ALK, ROS1, RET), single‐gene assays (e.g., Sanger sequencing, real‐time polymerase chain reaction, fully automated and integrated testing platforms for EGFR, KRAS, BRAF mutations), and multiplex, high‐throughput sequencing platforms (e.g., NGS). Testing strategies include sequential assays (either methodology‐based or gene‐based) or panel‐based assays. Multiplexed assays may be small panels (≤50 genes) or expanded panels (with several hundred genes that encompass most of the known somatic changes in solid tumors). It is worth noting that there is inherent conflict between comprehensive biomarker testing and the TAT of results. Various forms of noncomprehensive biomarker testing may be more easily performed than comprehensive biomarker testing. We advocate for comprehensive assessment of actionable mutations to direct patient treatment decisions whenever possible.
Sequential testing, in which a biomarker (gene‐based) or panel (methodology‐based) is tested stepwise before proceeding to other tests, has been judged by many practices to be convenient and cost‐effective. Nevertheless, this strategy is falling out of favor because of the expanding number of biomarkers that need to be assessed promptly. 57 Some sequential testing strategies may be preferred based on their short TAT, making them alluring when there is clinical urgency to start treatment. For instance, IHC‐based and FISH‐based test results are typically available within a few days, unlike NGS test results, which frequently take 2 or more weeks (Table 2). A sequential testing approach requires individual practice‐specific standard operating procedures that prioritize one particular gene or assay over another. However, a sequential single‐gene testing approach may exhaust the tumor tissue because each time a tissue block is recut for sections for additional testing, the block needs to be refaced, thus losing precious tissue material and raising the risk of tissue exhaustion. One study evaluating the adequacy of small biopsy specimens for lung cancer biomarker testing demonstrated that only 18% of patients had samples that were adequate for comprehensive testing of all guideline‐recommended biomarkers when using a single‐gene testing approach. 15 In addition, waiting for each sequential test to be complete before moving to the next can consume valuable time. 58
TABLE 2.
The types of biomarker testing available that guide the choice of the testing platform.
| Testing method | Testing biomarkers | Turnaround time | Testing priority | |
|---|---|---|---|---|
| Single‐gene assays | Molecular (e.g., Sanger sequencing, real‐time PCR) | EGFR, KRAS, BRAF, ERBB2, ALK, ROS1, RET, MET, NTRK1–3 | 5–10 days | If performed sequentially, increases the overall turnaround time of test results and depletes tissue |
| FISH | ALK, ROS1, RET, MET, a NTRK1–3 | 5–7 days | ||
| IHC | ALK, ROS1, b MET, c PD‐L1 | 2–5 days | ||
| Panel‐based, multiplexed assays | Molecular e.g. NGS | EGFR, KRAS, BRAF, ERBB2, ALK, ROS1, RET, MET, NTRK1–3 | ≤10 days (Lindeman 2013 29 ) | Minimizes tissue wastage and optimizes overall turnaround time of test results |
Abbreviations: FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next‐generation sequencing; PCR, polymerase chain reaction; PD‐L1, programmed death‐ligand 1.
FISH can detect MET copy number changes but not MET exon 14‐skipping mutations.
ROS1 fusions detected by IHC require confirmation by molecular testing/FISH.
IHC can detect MET amplification but not MET exon 14‐skipping mutations.
The CAP/IASLC/AMP guidelines recommend that laboratories consider cutting multiple additional unstained sections up‐front for biomarker testing to minimize the need to reface the tissue block and prevent tissue loss during processing in the histology laboratory. 29 Furthermore, having sections available to submit for biomarker studies when finalizing the diagnosis can reduce TAT because there is minimal delay in waiting for the block to be recut.
Historically, molecular laboratories have prioritized single‐gene assays because billing for these assays and reimbursement for testing are more consistent. Furthermore, many IHC‐based assays (PD‐L1, ALK, ROS1) are performed in‐house and are preferred for this reason. Panel‐based testing provides results for all guideline‐recommended biomarkers and a more predictable TAT for all, including relatively uncommon mutations. Therefore, the lower cost and relative ease of using single‐gene testing may be an attractive approach to some practices (especially when comprehensive NGS assays require integration of laboratory processes with an outside vendor, seem cost‐prohibitive, or raise concerns about reimbursement). In reality, primary reliance on a single‐gene testing approach poses a higher risk of depleting limited tissue material and failing to provide results for less prevalent biomarkers that are lower on the sequential testing priority list.
When choosing a testing platform, the goal for all laboratories should be to adhere to the CAP/IASLC/AMP guidelines, which recommend a TAT of ≤10 working days for tissue‐based molecular testing results, while comprehensively testing all guideline‐recommended biomarkers for individual patients. 2 Ultimately, the choice of a testing platform that is optimal for patient care within an individual practice and provides biomarker test results with an acceptable TAT will depend on institutional infrastructure, access to comprehensive testing resources, and input from physicians involved in clinical decision making.
NGS‐based testing requires multiple steps (nucleic acid extraction and purification, library preparation, sequencing, analysis using a bioinformatics pipeline) and typically takes 2 weeks or longer. The TAT can vary significantly within molecular testing platforms based on the method used for sample purification and sequencing. Also, laboratories need to decide on DNA‐based versus RNA‐based NGS assays for the detection of gene fusions because there are some inherent limitations related to DNA‐based, amplicon‐mediated testing. This may lead to adding days to the TAT if a sequential NGS strategy (RNA‐based testing for gene fusions, if initial DNA‐based NGS is negative for actionable targets) is adopted. The emergence of integrated NGS solutions can considerably reduce the TAT to a few days (approximately 3 days), even for large, expanded panels; however, cost and throughput may serve as barriers to widespread implementation of these solutions. 59 , 60 However, no test is perfect. Examining a biomarker with multiple modalities (for instance, ALK detection by FISH, IHC, or NGS) may increase the likelihood of detecting common alterations, especially for cases in which molecular alterations may be missed because of low specimen tumor content.
Type of testing laboratory and tissue‐handling processes
The choice of in‐house testing versus send‐out testing by a reference laboratory is an institutional decision. It is usually based on multiple factors, including test volume, direct and indirect costs, available resources, infrastructure for test performance, validation and operations, regulations related to testing, such as patents and licenses, and reimbursement. Specific workflows need to be established for material to be sent out for testing or used for in‐house assays. If biomarker testing is being sent to different reference laboratories for separate assays (e.g., PD‐L1 IHC and NGS for mutations and gene fusions), practices must consider specimen requirements and acceptance criteria for each assay to minimize delays in test reporting, including inability to test because of sample inadequacy. An efficient workflow to handle tissue requests for biomarker testing (molecular, FISH, and IHC) can reduce the time from specimen procurement and diagnosis to tissue submission for biomarker testing, thus improving overall TAT. Rapid molecular tests that provide results within hours to days may be considered for the more frequent gene alterations and expanded NGS assays for comprehensive testing. 61 , 62 , 63 However, this approach needs to be evaluated within the context of tissue availability, resources, and reimbursement considerations before implementation.
Incorporating the plasma genotyping option
Contemporary management of metastatic NSCLC hinges on comprehensive biomarker testing. Yet, in multiple studies, 20% to 30% of patients with newly diagnosed NSCLC do not have EGFR or ALK genotyping results, let alone the full range of guideline‐recommended biomarkers, before initiating therapy. 15 The MYLUNG (Molecularly Informed Lung Cancer Treatment in a Community Cancer Network) consortium study, which evaluated real‐world biomarker testing rates for five biomarkers (EGFR, ALK, ROS1, BRAF, and PD‐L1), reported that, although most patients with metastatic NSCLC received at least one biomarker test before first‐line systemic therapy, <50% received testing for all five biomarkers. 64 Plasma‐based genotyping assays (also frequently referred to as liquid biopsy) have a high positive predictive value, may provide actionable results, and expedite clinical decisions if a targetable alteration is detected. However, the sensitivity of plasma‐based genotyping assays may be suboptimal in patients with a lower metastatic tumor burden. Additional limitations include lack of assessment of PD‐L1 expression to direct immunotherapy use and lower performance to detect certain targetable mutations (e.g., fusions and copy number variants). Nevertheless, these assays are useful when positive but should prompt further tissue testing when negative, and a more comprehensive approach to tumor genotyping is preferred.
Although CAP/IASLC/AMP molecular testing guidelines recommend plasma‐based genotyping assays as complementary to (not a replacement for) tissue‐based testing, 2 recent US Food and Drug Administration approvals of commercial assays support plasma‐based genotyping for up‐front tumor profiling. False‐negative results remain a limitation, and practitioners must recognize the ongoing need for tissue‐based testing. However, given the pressure for expeditious comprehensive genotyping results, plasma‐based genotyping assays with shorter TAT (approximately 5–7 days) and easy access to specimens can be attractive. 15 Therefore, a possible workflow could involve performing plasma‐based genotyping on all patients with metastatic NSCLC at the time of their initial oncology appointment, with tissue testing either concurrently or subsequently if the liquid biopsy is negative. 65 This latter strategy can further delay access to actionable biomarker data. Unfortunately, at this time, most third‐party payors will not cover both plasma‐based genotyping and tumor tissue‐based testing for patients with NSCLC, thereby limiting routine implementation of such an approach. This may change in time with the accumulation of evidence.
Assessing quality metrics and tools to incorporate feedback
Performance benchmarking is critical to improving TAT, closely monitoring quality metrics, and using process‐improvement tools to identify areas that may benefit from additional resources (Figure 1). 29 , 55 , 65 Testing algorithms and decision aids for choosing the optimal biomarker testing strategy can help reduce the time to test initiation and improve key metrics (Table 3). Developing evidence‐based clinical decision tools that associate biomarker test results with specific treatment algorithms, dissemination efforts for educating physicians on testing strategies, and creating tools, such as dashboards, that can provide real‐time aggregate monitoring of biomarker test ordering and reporting may improve testing TAT and decision making.
FIGURE 1.
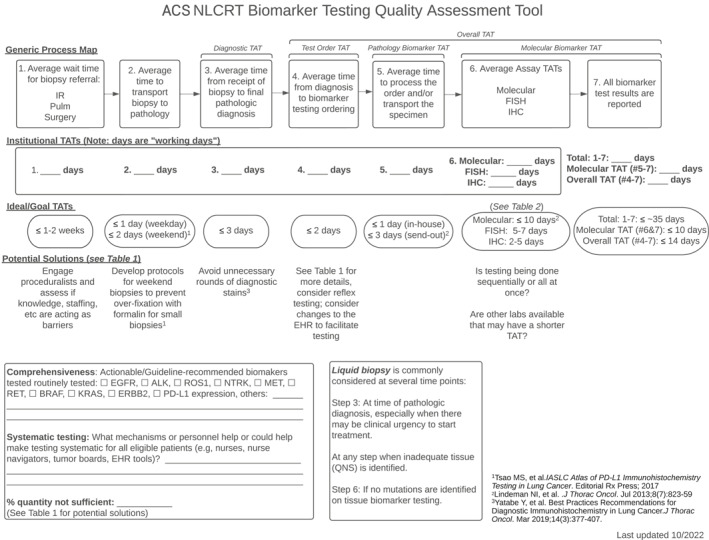
Non–small cell lung cancer biomarker testing quality‐assessment tool illustrating the workflow for specimen diagnosis and biomarker testing. Included are steps to identifying delays in TAT and solutions that can help improve the process. EHR indicates electronic health record; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; IR, interventional radiology; ACS NLCRT, American Cancer Society National Lung Cancer Roundtable; TATs, turnaround times; PD‐L1, programmed death‐ligand 1; Pulm, pulmonary; QNS, quantity not sufficient. 29 , 55 , 65
TABLE 3.
Key quality metrics that can guide quality‐improvement efforts.
| Proposed quality metric | 90% compliance goal |
|---|---|
| Pathology diagnostic turnaround time (i.e., time from specimen received in pathology to final pathologic diagnosis) | ≤3 working days |
| Biomarker test order turnaround time (i.e., time from final pathologic diagnosis to biomarker test ordered) | ≤2 working days |
| Pathology biomarker turnaround time (i.e., time from final pathologic diagnosis and/or biomarker test ordered to specimen sent to molecular laboratory) for eligible patients with NSCLC | ≤3 working days (Lindeman 2013 29 ) |
| Molecular biomarker turnaround time (i.e., time from specimen received in molecular testing laboratory to reporting of all biomarker results) for eligible patients with NSCLC | ≤10 working days (Lindeman 2013 29 ) |
| Overall biomarker turnaround time (i.e., time from final pathologic diagnosis rendered to reporting of all biomarker results) for eligible patients with NSCLC | ≤14 working days |
Abbreviation: NSCLC, non–small cell lung cancer.
CONCLUSION
Reducing TAT for comprehensive biomarker testing in NSCLC to fully inform treatment decisions is challenging but necessary. Given the diverse clinical scenarios, practice settings, and logistical barriers, there are different effective approaches to establishing timely access to biomarker information. The goal is to achieve timely, comprehensive biomarker testing for therapeutic decision making, given the rapid expansion of biomarker‐driven, personalized NSCLC treatment. Minimizing test ordering delays should be a key institutional, practice, and physician objective. Frequent interdisciplinary communication and collaboration, leveraging tools available in the EHR to aid in ordering and notification of results, and developing standardized protocols for test orders are critical elements to consider.
In summary, reducing TAT needs to be a multidisciplinary effort. It cannot be accomplished unless there is consensus and coordination of standard operating procedures, roles, and relationships among all key specialties within the institution. Ensuring adequate tissue collection, optimizing specimen handling, and processing to maximize tissue preservation for biomarker testing can improve patient access to comprehensive biomarker testing. The transition from sequential, single‐gene testing to reflexive, panel‐based molecular testing, the complementary use of plasma‐based genotyping, and the use of rapid molecular assays can also expand access to timely, comprehensive genotyping. Finally, a robust quality‐assurance program is vital to monitor implementation success. Expanding access to timely, comprehensive biomarker testing will remain an iterative process given the rapid evolution in the field. Existing processes must be adaptable to new knowledge and clinical relevance, including evolving eligibility criteria and technological advances that change the optimal workflow. They should incorporate tools for assessing program implementation across the spectrum of health care delivery systems. The ultimate goal is to foster universal implementation of timely, comprehensive biomarker testing to promote universal access to precision cancer medicine for all patients with lung cancer.
AUTHOR CONTRIBUTIONS
Sinchita Roy‐Chowdhuri: Conceptualization, formal analysis, project administration, writing–original draft preparation, and review and editing. Haresh Mani: Formal analysis, writing–original draft preparation, and review and editing. Adam H. Fox: Formal analysis, writing–original draft preparation, and review and editing. Anne Tsao: Conceptualization and writing–review and editing. Lynette M. Sholl: Conceptualization and writing–review and editing. Farhood Farjah: Writing–review and editing. Bruce E. Johnson: Writing–review and editing. Raymond U. Osarogiagbon: Writing–review and editing. M. Patricia Rivera: Writing–review and editing. Gerard A. Silvestri: Writing–review and editing. Robert A. Smith: Resources and writing–review and editing. Ignacio I. Wistuba: Conceptualization, supervision, and writing–review and editing.
CONFLICT OF INTEREST STATEMENT
Sinchita Roy‐Chowdhuri serves on the Executive Board of the American Society of Cytopathology and is an Associate Editor for Cancer Cytopathology. Haresh Mani reports travel support from the American Cancer Society. Adam H. Fox reports a grant from the National Institutes of Health (K12‐CA157688) during the course of the study; consulting fees from Amgen and the Association of Community Cancer Centers; grants from the American Cancer Society and Hollings Medical Center, Medical University of South Carolina; and owns stock in Merck outside the submitted work. Anne Tsao reports consulting fees from Ariad Pharmaceuticals, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Merck, Novartis, Pfizer, Roche, and Seattle Genetics outside the submitted work. Lynette M. Sholl is a consultant for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Genentech, and GV20 Therapeutics, all outside the submitted work. Bruce E. Johnson reports postmarketing royalties from the Dana‐Farber Cancer Institute for EGFR mutation testing and is a consultant for Abdera Therapeutics, the American Cancer Society, AstraZeneca, Bluedot Bio, Checkpoint Therapeutics, Daiichi Sankyo, G1 Therapeutics, Genentech, GlaxoSmithKline, Hummingbird Diagnostics, Jazz Pharmaceuticals, Merus NV, and Novartis, all outside the submitted work. Raymond U. Osarogiagbon owns patents for a lymph node specimen‐collection kit; owns stock in Eli Lilly, Gilead Sciences, and Pfizer; has worked as a paid research consultant for Astra Zeneca; and serves as board chair of the Hope Foundation for Cancer Research, all outside the submitted work. M. Patricia Rivera is a consultant for the American Board of Internal Medicine and the American Cancer Society and serves as president of the American Thoracic Society. Gerard A. Silvestri is a consultant for Olympus America outside the submitted work. Robert A. Smith reports that the American Cancer Society receives unrestricted educational funding from AbbVie, Amgen, AstraZeneca, Daiichi‐Sankyo, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Foundation Medicine, Genentech, Guardant Health, Johnson & Johnson, Merck, Novartis, Novocure, Regeneron, Roche, Sanofi‐Genzyme, Takeda, and in‐kind support from the American Cancer Society. Ignacio I. Wistuba reports grants and personal fees from Amgen, AstraZeneca, Daiichi Sankyo, Genentech, Novartis, and Pfizer; grants from 4D, Adaptimmune, Bayer, Bristol‐Myers Squibb, EMD Serono, Iovance, Johnson & Johnson, Karus, Merck, Sanofi, and Takeda; and personal fees from Flame, Guardant Health, Merus, Oncocyte, Regeneron, and Roche, all outside the submitted work. Farhood Farjah disclosed no conflicts of interest.
ACKNOWLEDGMENTS
The ACS receives funding support for the ACS NLCRT from AbbVie, Amgen, AstraZeneca, Daiichi‐Sankyo, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Foundation Medicine, Genentech, Guardant Health, Johnson & Johnson, Merck, Novartis, Novocure, Regeneron, Roche, Sanofi‐Genzyme, and Takeda. Role of sponsors: The sponsors had no role in the design of the study, the collection, and analysis of the data, or the preparation of the manuscript.
Roy‐Chowdhuri S, Mani H, Fox AH, et al. The American Cancer Society National Lung Cancer Roundtable strategic plan: methods for improving turnaround time of comprehensive biomarker testing in non–small cell lung cancer. Cancer. 2024;130(24):4200‐4212. doi: 10.1002/cncr.34926
REFERENCES
- 1. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) . Non‐Small Cell Lung Cancer. Version 3.2022. Accessed April 26, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 2. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142(3):321‐346. doi: 10.5858/arpa.2017-0388-cp [DOI] [PubMed] [Google Scholar]
- 3. Kalemkerian GP, Narula N, Kennedy EB. Molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement summary of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Oncol Pract. 2018;14(5):323‐327. doi: 10.1200/jop.18.00035 [DOI] [PubMed] [Google Scholar]
- 4. Planchard D, Popat S, Kerr K, et al. Metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(suppl 4):iv192‐iv237. doi: 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 5. Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36(9):911‐919. doi: 10.1200/jco.2017.76.7293 [DOI] [PubMed] [Google Scholar]
- 6. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. doi: 10.1056/nejmoa1801005 [DOI] [PubMed] [Google Scholar]
- 7. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018‐2028. doi: 10.1056/nejmoa1501824 [DOI] [PubMed] [Google Scholar]
- 8. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819‐1830. doi: 10.1016/s0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1–positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. doi: 10.1056/nejmoa1606774 [DOI] [PubMed] [Google Scholar]
- 10. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science. 2015;348(6230):124‐128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berghoff AS, Bellosillo B, Caux C, et al. Immune checkpoint inhibitor treatment in patients with oncogene‐addicted non‐small cell lung cancer (NSCLC): summary of a multidisciplinary round‐table discussion. ESMO Open. 2019;4(3):e000498. doi: 10.1136/esmoopen-2019-000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR‐TKI‐associated interstitial pneumonitis in nivolumab‐treated patients with non‐small cell lung cancer. JAMA Oncol. 2018;4(8):1112‐1115. doi: 10.1001/jamaoncol.2017.4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585‐4593. doi: 10.1158/1078-0432.ccr-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mileham KF, Schenkel C, Bruinooge SS, et al. Defining comprehensive biomarker‐related testing and treatment practices for advanced non‐small‐cell lung cancer: results of a survey of U.S. oncologists. Cancer Med. 2022;11(2):530‐538. doi: 10.1002/cam4.4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell‐free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non‐small cell lung cancer. Clin Cancer Res. 2019;25(15):4691‐4700. doi: 10.1158/1078-0432.ccr-19-0624 [DOI] [PubMed] [Google Scholar]
- 16. Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non‐small cell lung cancer: optimizing the diagnostic journey. Transl Lung Cancer Res. 2019;8(3):286‐301. doi: 10.21037/tlcr.2019.04.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non‐small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651‐659. doi: 10.1016/j.cllc.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 18. Dalurzo ML, Aviles‐Salas A, Soares FA, et al. Testing for EGFR mutations and ALK rearrangements in advanced non‐small‐cell lung cancer: considerations for countries in emerging markets. Onco Targets Ther. 2021;14:4671‐4692. doi: 10.2147/ott.s313669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smeltzer MP, Wynes MW, Lantuejoul S, et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol. 2020;15(9):1434‐1448. doi: 10.1016/j.jtho.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 20. Lim C, Tsao MS, Le LW, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall‐cell lung cancer. Ann Oncol. 2015;26(7):1415‐1421. doi: 10.1093/annonc/mdv208 [DOI] [PubMed] [Google Scholar]
- 21. Singh N, Temin S, Baker S, et al. Therapy for stage IV non‐small‐cell lung cancer without driver alterations: ASCO living guideline. J Clin Oncol. 2022;40(28):3323‐3343. doi: 10.1200/jco.22.00825 [DOI] [PubMed] [Google Scholar]
- 22. Singh N, Temin S, Baker S, et al. Therapy for stage IV non‐small‐cell lung cancer with driver alterations: ASCO living guideline. J Clin Oncol. 2022;40(28):3310‐3322. doi: 10.1200/jco.22.00824 [DOI] [PubMed] [Google Scholar]
- 23. Meric‐Bernstam F, Brusco L, Shaw K, et al. Feasibility of large‐scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol. 2015;33(25):2753‐2762. doi: 10.1200/jco.2014.60.4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non‐small‐cell lung cancer: results of a 1‐year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415‐1426. doi: 10.1016/s0140-6736(16)00004-0 [DOI] [PubMed] [Google Scholar]
- 25. Anand K, Phung TL, Bernicker EH, Cagle PT, Olsen RJ, Thomas JS. Clinical utility of reflex ordered testing for molecular biomarkers in lung adenocarcinoma. Clin Lung Cancer. 2020;21(5):437‐442. doi: 10.1016/j.cllc.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 26. DiStasio M, Chen Y, Rangachari D, Costa DB, Heher YK, VanderLaan PA. Molecular testing turnaround time for non‐small cell lung cancer in routine clinical practice confirms feasibility of CAP/IASLC/AMP guideline recommendations: a single‐center analysis. Clin Lung Cancer. 2017;18(5):e349‐e356. doi: 10.1016/j.cllc.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 27. VanderLaan PA, Chen Y, DiStasio M, Rangachari D, Costa DB, Heher YK. Molecular testing turnaround time in non‐small‐cell lung cancer: monitoring a moving target. Clin Lung Cancer. 2018;19(5):e589‐e590. doi: 10.1016/j.cllc.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 28. Pichler T, Rohrmoser A, Letsch A, et al. Information, communication, and cancer patients' trust in the physician: what challenges do we have to face in an era of precision cancer medicine? Support Care Cancer. 2021;29(4):2171‐2178. doi: 10.1007/s00520-020-05692-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137(7):828‐860. doi: 10.1097/jto.0b013e318290868f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spiegel ML, Goldman JW, Wolf BR, et al. Non‐small cell lung cancer clinical trials requiring biopsies with biomarker‐specific results for enrollment provide unique challenges. Cancer. 2017;123(24):4800‐4807. doi: 10.1002/cncr.31056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim C, Sung M, Shepherd FA, et al. Patients with advanced non‐small cell lung cancer: are research biopsies a barrier to participation in clinical trials? J Thorac Oncol. 2016;11(1):79‐84. doi: 10.1016/j.jtho.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 32. Tsimberidou AM, Elkin S, Dumanois R, Pritchard D. Clinical and economic value of genetic sequencing for personalized therapy in non‐small‐cell lung cancer. Clin Lung Cancer. 2020;21(6):477‐481. doi: 10.1016/j.cllc.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 33. Zer A, Cutz JC, Sekhon H, et al. Translation of knowledge to practice‐improving awareness in NSCLC molecular testing. J Thorac Oncol. 2018;13(7):1004‐1011. doi: 10.1016/j.jtho.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 34. Fox AH, Jett JR, Roy UB, et al. Knowledge and practice patterns among pulmonologists for molecular biomarker testing in advanced non‐small cell lung cancer. Chest. 2021;160(6):2293‐2303. doi: 10.1016/j.chest.2021.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snell R (ed.). J Health Care Compl. September‐October 2018; (SKU:10041602), 20: 21‐32; 59‐60. CCH and Wolters Kluwer. https://law‐store.wolterskluwer.com/s/product/journal‐of‐health‐care‐compliance‐vitallaw‐3r/01tG000000LtyotIAB
- 36. Center for Medicare and Medicaid Services (CMS.gov) . Laboratory Date of Service Policy. Accessed April 6, 2022. https://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/ClinicalLabFeeSched/Clinical‐Lab‐DOS‐Policy
- 37. Zacharias M, Absenger G, Kashofer K, et al. Reflex testing in non‐small cell lung carcinoma using DNA‐ and RNA‐based next‐generation sequencing‐a single‐center experience. Transl Lung Cancer Res. 2021;10(11):4221‐4234. doi: 10.21037/tlcr-21-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364‐1382. doi: 10.5858/arpa.2018-0902-sa [DOI] [PubMed] [Google Scholar]
- 39. Miller RJ, Mudambi L, Vial MR, Hernandez M, Eapen GA. Evaluation of appropriate mediastinal staging among endobronchial ultrasound bronchoscopists. Ann Am Thorac Soc. 2017;14:1162‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheema PK, Menjak IB, Winterton‐Perks Z, et al. Impact of reflex EGFR/ALK testing on time to treatment of patients with advanced nonsquamous non‐small‐cell lung cancer. J Oncol Pract. 2017;13(2):e130‐e138. doi: 10.1200/jop.2016.014019 [DOI] [PubMed] [Google Scholar]
- 41. Cheema PK, Raphael S, El‐Maraghi R, et al. Rate of EGFR mutation testing for patients with nonsquamous non‐small‐cell lung cancer with implementation of reflex testing by pathologists. Curr Oncol. 2017;24(1):16‐22. doi: 10.3747/co.24.3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doxtader EE, Cheng YW, Zhang Y. Molecular testing of non‐small cell lung carcinoma diagnosed by endobronchial ultrasound‐guided transbronchial fine‐needle aspiration. Arch Pathol Lab Med. 2019;143(6):670‐676. doi: 10.5858/arpa.2017-0184-RA [DOI] [PubMed] [Google Scholar]
- 43. Tian SK, Killian JK, Rekhtman N, et al. Optimizing workflows and processing of cytologic samples for comprehensive analysis by next‐generation sequencing: Memorial Sloan Kettering Cancer Center experience. Arch Pathol Lab Med. 2016;140(11):1200‐1205. doi: 10.5858/arpa.2016-0108-ra [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roy‐Chowdhuri S, Chen H, Singh RR, et al. Concurrent fine needle aspirations and core needle biopsies: a comparative study of substrates for next‐generation sequencing in solid organ malignancies. Mod Pathol. 2017;30(4):499‐508. doi: 10.1038/modpathol.2016.228 [DOI] [PubMed] [Google Scholar]
- 45. Hwang DH, Garcia EP, Ducar MD, Cibas ES, Sholl LM. Next‐generation sequencing of cytologic preparations: an analysis of quality metrics. Cancer. 2017;125(10):786‐794. doi: 10.1002/cncy.21897 [DOI] [PubMed] [Google Scholar]
- 46. Roy‐Chowdhuri S, Aisner DL, Allen TC, et al. Biomarker testing in lung carcinoma cytology specimens: a perspective from members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 2016;140(11):1267‐1272. doi: 10.5858/arpa.2016-0091-sa [DOI] [PubMed] [Google Scholar]
- 47. Roh MH. The utilization of cytologic fine‐needle aspirates of lung cancer for molecular diagnostic testing. J Pathol Transl Med. 2015;49(4):300‐309. doi: 10.4132/jptm.2015.06.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bellevicine C, Malapelle U, Vigliar E, Pisapia P, Vita G, Troncone G. How to prepare cytological samples for molecular testing. J Clin Pathol. 2017;70(10):819‐826. doi: 10.1136/jclinpath-2017-204561 [DOI] [PubMed] [Google Scholar]
- 49. Trisolini R, Cancellieri A, Tinelli C, et al. Randomized trial of endobronchial ultrasound‐guided transbronchial needle aspiration with and without rapid on‐site evaluation for lung cancer genotyping. Chest. 2015;148(6):1430‐1437. doi: 10.1378/chest.15-0583 [DOI] [PubMed] [Google Scholar]
- 50. Roy‐Chowdhuri S, Dacic S, Ghofrani M, et al. Collection and handling of thoracic small biopsy and cytology specimens for ancillary studies: guideline from the College of American Pathologists in collaboration with the American College of Chest Physicians, Association for Molecular Pathology, American Society of Cytopathology, American Thoracic Society, Pulmonary Pathology Society, Papanicolaou Society of Cytopathology, Society of Interventional Radiology, and Society of Thoracic Radiology. Arch Pathol Lab Med. 2020;144(8):933‐958. doi: 10.5858/arpa.2020-0119-cp [DOI] [PubMed] [Google Scholar]
- 51. VanderLaan PA, Chen Y, Alex D, et al. Results from the 2019 American Society of Cytopathology survey on rapid on‐site evaluation—Part 1: objective practice patterns. J Am Soc Cytopathol. 2019;8(6):333‐341. doi: 10.1016/j.jasc.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 52. Padmanabhan V, Steinmetz HB, Rizzo EJ, et al. Improving adequacy of small biopsy and fine‐needle aspiration specimens for molecular testing by next‐generation sequencing in patients with lung cancer: a quality improvement study at Dartmouth‐Hitchcock Medical Center. Arch Pathol Lab Med. 2017;141(3):402‐409. doi: 10.5858/arpa.2016-0096-oa [DOI] [PubMed] [Google Scholar]
- 53. Voyten J, Holtzman MP, Pantanowitz L, et al. Lessons learned from clinical trial queries on small biopsy collections: importance of rapid on‐site evaluation. J Am Soc Cytopathol. 2020;9(5):461‐468. doi: 10.1016/j.jasc.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 54. Aisner DL, Rumery MD, Merrick DT, et al. Do more with less: tips and techniques for maximizing small biopsy and cytology specimens for molecular and ancillary testing: the University of Colorado experience. Arch Pathol Lab Med. 2016;140(11):1206‐1220. doi: 10.5858/arpa.2016-0156-ra [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yatabe Y, Dacic S, Borczuk AC, et al. Beencer. J Thorac Oncol. 2019;14(3):377‐407. doi: 10.1016/j.jtho.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen H, Luthra R, Goswami RS, Singh RR, Roy‐Chowdhuri S. Analysis of pre‐analytic factors affecting the success of clinical next‐generation sequencing of solid organ malignancies. Cancers (Basel). 2015;7(3):1699‐1715. doi: 10.3390/cancers7030859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Layfield LJ, Hammer RD, White SK, Furtado LV, Schmidt RL. Molecular testing strategies for pulmonary adenocarcinoma: an optimal approach with cost analysis. Arch Pathol Lab Med. 2019;143(5):628‐633. doi: 10.5858/arpa.2018-0218-oa [DOI] [PubMed] [Google Scholar]
- 58. Pennell NA, Mutebi A, Zhou ZY, et al. Economic impact of next‐generation sequencing versus single‐gene testing to detect genomic alterations in metastatic non‐small‐cell lung cancer using a decision analytic model. JCO Precis Oncol. 2019;3:1‐9. doi: 10.1200/po.18.00356 [DOI] [PubMed] [Google Scholar]
- 59. Sheffield BS, Beharry A, Diep J, et al. Point of care molecular testing: community‐based rapid next‐generation sequencing to support cancer care. Curr Oncol. 2022;29(3):1326‐1334. doi: 10.3390/curroncol29030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ilie M, Hofman V, Bontoux C, et al. Setting up an ultra‐fast next‐generation sequencing approach as reflex testing at diagnosis of non‐squamous non‐small cell lung cancer; experience of a single center (LPCE, Nice, France). Cancers (Basel). 2022;14(9):2258. doi: 10.3390/cancers14092258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Caputo A, D'Ardia A, Sabbatino F, et al. Testing EGFR with Idylla on cytological specimens of lung cancer: a review. Int J Mol Sci. 2021;22(9):4852. doi: 10.3390/ijms22094852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hawkins P, Stevenson T, Powari M. Use of the Idylla EGFR mutation test for variant detection in non‐small cell lung cancer samples. Am J Clin Pathol. 2021;156(4):653‐660. doi: 10.1093/ajcp/aqab003 [DOI] [PubMed] [Google Scholar]
- 63. Ilie M, Butori C, Lassalle S, et al. Optimization of EGFR mutation detection by the fully‐automated qPCR‐based Idylla system on tumor tissue from patients with non‐small cell lung cancer. Oncotarget. 2017;8(61):103055‐103062. doi: 10.18632/oncotarget.21476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robert NJ, Espirito JL, Chen L, et al. Biomarker testing and tissue journey among patients with metastatic non‐small cell lung cancer receiving first‐line therapy in the US Oncology Network. Lung Cancer. 2022;166:197‐204. doi: 10.1016/j.lungcan.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 65. Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non‐small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. 2018;13(9):1248‐1268. doi: 10.1016/j.jtho.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 66. Tsao MS, Kerr KM, Dacic S, Yatabe Y, Hirsch FR, eds. IASLC Atlas of PD‐L1 Immunohistochemistry Testing in Lung Cancer. International Association for the Study of Lung Cancer (IASLC); 2017. [Google Scholar]


