Abstract
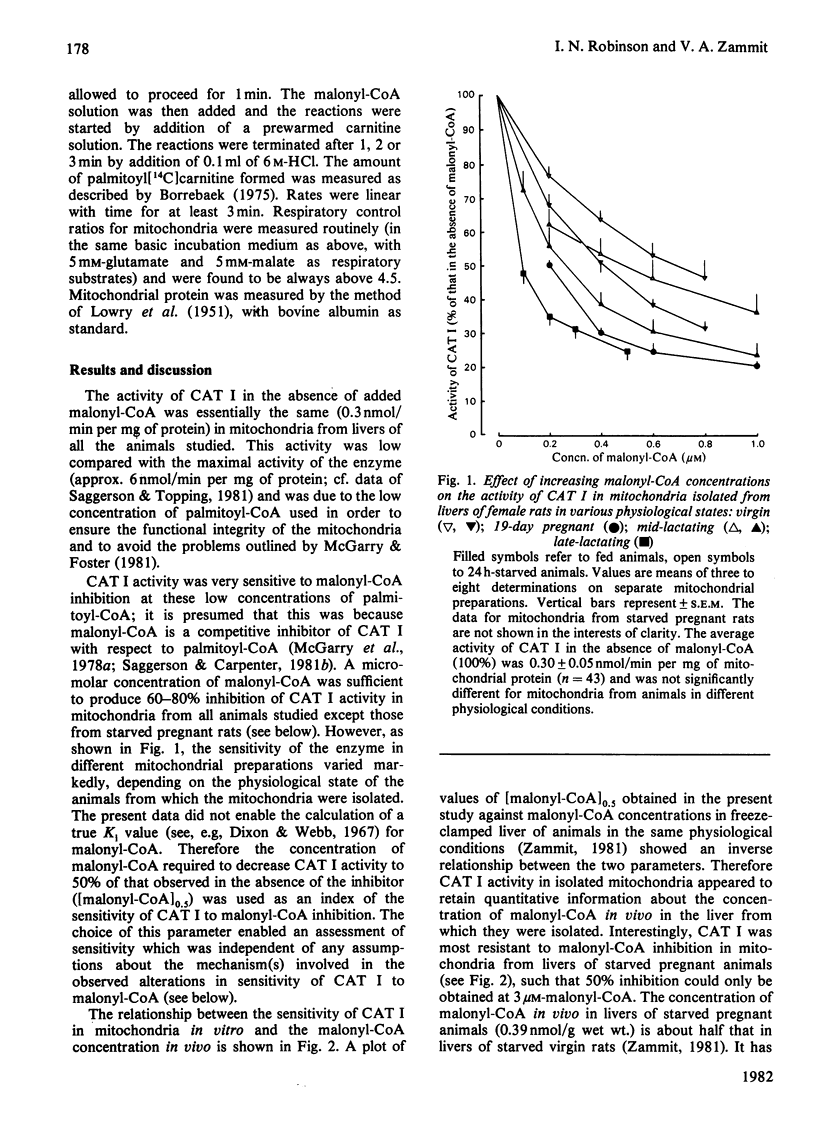
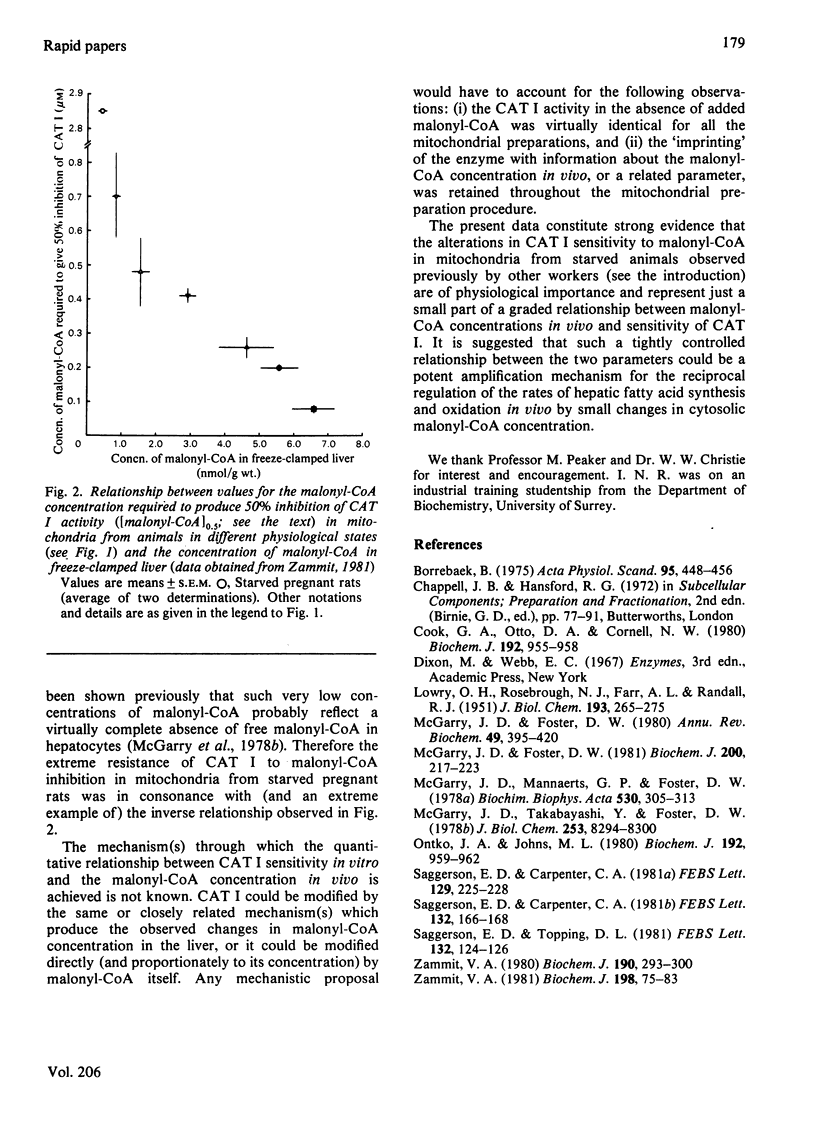
The sensitivity of carnitine acyltransferase I (EC 2.3.1.21) activity to malonyl-CoA inhibition in rat liver mitochondria isolated from animals in various physiological states was quantitatively proportional to the hepatic malonyl-CoA concentration in vivo. It is suggested that this relationship between the two parameters could result in a potent amplification mechanism for the reciprocal regulation of fatty acid synthesis and oxidation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borrebaek B. Acylation of carnitine and glycerophosphate in suspensions of rat liver mitochondria at varying levels of palmitate and coenzyme A. Acta Physiol Scand. 1975 Dec;95(4):448–456. doi: 10.1111/j.1748-1716.1975.tb10073.x. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Importance of experimental conditions in evaluating the malonyl-CoA sensitivity of liver carnitine acyltransferase. Studies with fed and starved rats. Biochem J. 1981 Nov 15;200(2):217–223. doi: 10.1042/bj2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. Characteristics of fatty acid oxidation in rat liver homogenates and the inhibitory effect of malonyl-CoA. Biochim Biophys Acta. 1978 Sep 28;530(3):305–313. doi: 10.1016/0005-2760(78)90150-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J. 1981 Jul 15;198(1):75–83. doi: 10.1042/bj1980075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. The effect of glucagon treatment and starvation of virgin and lactating rats on the rates of oxidation of octanoyl-L-carnitine and octanoate by isolated liver mitochondria. Biochem J. 1980 Aug 15;190(2):293–300. doi: 10.1042/bj1900293. [DOI] [PMC free article] [PubMed] [Google Scholar]


