Abstract
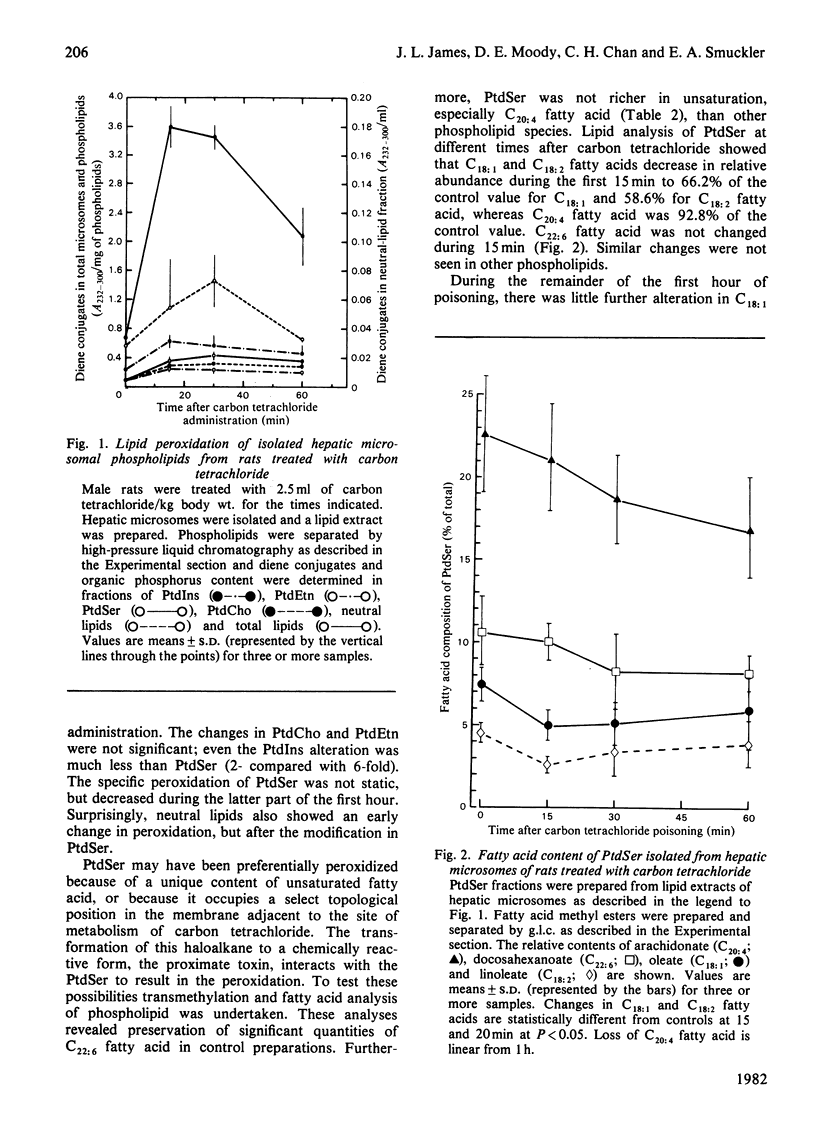
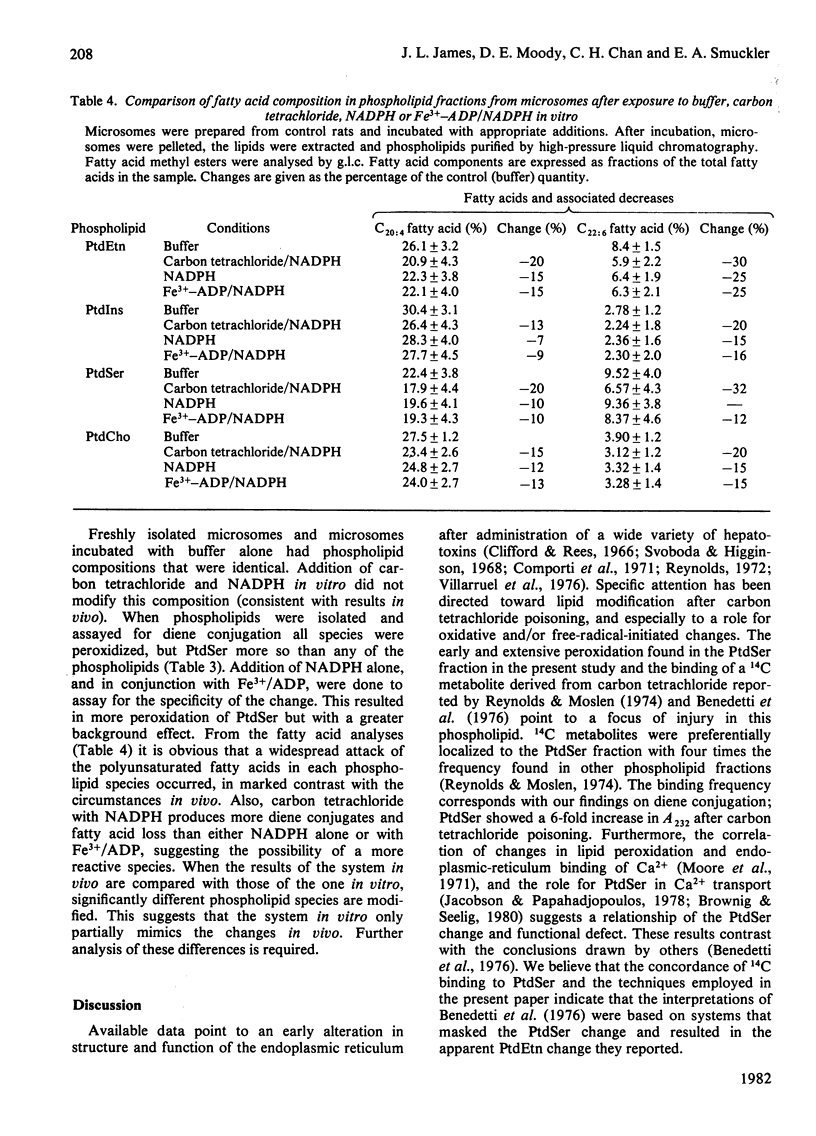
Endoplasmic-reticulum phospholipids were measured during the first hour after carbon tetrachloride administration to male Sprague–Dawley rats and compared with carbon tetrachloride challenge of microsomes from control animals in vitro. The extracted lipids were separated by high-pressure liquid chromatography. No significant differences in the abundance of phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol or phosphatidylcholine were found after either treatment when compared with untreated controls. Diene conjugate formation in each separated phospholipid was determined by measuring A232 and expressed on the basis of lipid phosphorus. Phosphatidylserine was peroxidized 6-fold greater than in controls after challenge in vivo, reaching maximal change after 15min, whereas the other phospholipids showed little or no alteration. Fatty acid composition analysis was performed by g.l.c. after transesterification of individual phospholipids. Phosphatidylserine revealed two types of response: an abrupt decrease in relative abundance of oleic acid (C18:1) and linoleic acid (C18:2) without further loss and a slower, linear decrease in arachidonic acid (C20:4) over the first hour. Similar changes were not seen in other phospholipids. In the `in vitro' model, the relative amounts of the phospholipids do not change. The extent of peroxidation was greater in all the phospholipids than found in vivo, with phosphatidylserine peroxidized to the greatest extent. These data suggest that carbon tetrachloride injury in vivo produces an early peroxidative event and that a specific phospholipid (phosphatidylserine) is selectively modified, although maintaining its relative concentration in the membrane. Dissection of this process in vitro will require refinement of existing systems to reduce the non-specific changes associated with the model system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Benedetti A., Casini A., Ferrali M., Comporti M. Characterization of the early molecular alterations of liver microsomal lipids following CCl4 intoxication. Panminerva Med. 1976 Nov-Dec;18(11-12):433–440. [PubMed] [Google Scholar]
- Browning J. L., Seelig J. Bilayers of phosphatidylserine: a deuterium and phosphorus nuclear magnetic resonance study. Biochemistry. 1980 Mar 18;19(6):1262–1270. doi: 10.1021/bi00547a034. [DOI] [PubMed] [Google Scholar]
- Clifford J., Rees K. R. Biochemical changes in the endoplasmic reticulum in liver injury. J Pathol Bacteriol. 1966 Jan;91(1):215–222. doi: 10.1002/path.1700910125. [DOI] [PubMed] [Google Scholar]
- Comporti M., Burdino E., Ugazio G. Alterazioni della composizione in acidi grassi dei lipidi del fegato e dei microsomi epatici nel ratto intossicato con tetracloruro di carbonio. Boll Soc Ital Biol Sper. 1969 May 31;45(10):700–703. [PubMed] [Google Scholar]
- Comporti M., Burdino E., Ugazio G. Changes in fatty acid pattern of liver microsomal phospholipids in rats treated with carbon tetrachloride. Ital J Biochem. 1971 Sep-Dec;20(5):156–165. [PubMed] [Google Scholar]
- Davison S. C., Wills E. D. Studies on the lipid composition of the rat liver endoplasmic reticulum after induction with phenobarbitone and 20-methylcholanthrene. Biochem J. 1974 Jun;140(3):461–468. doi: 10.1042/bj1400461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Dallner G. Lipid composition and turnover of rough and smooth microsomal membranes in rat liver. J Lipid Res. 1968 Nov;9(6):720–729. [PubMed] [Google Scholar]
- HORNING M. G., EARLE M. J., MALING H. M. Changes in fatty acid composition of liver lipids induced by carbon tetrachloride and ethionine. Biochim Biophys Acta. 1962 Jan 1;56:175–177. doi: 10.1016/0006-3002(62)90545-0. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975 Jan 14;14(1):152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- James J. L., Clawson G. A., Chan C. H., Smuckler E. A. Analysis of the phospholipid of the nuclear envelope and endoplasmic reticulum of liver cells by high pressure liquid chromatography. Lipids. 1981 Jul;16(7):541–545. doi: 10.1007/BF02535053. [DOI] [PubMed] [Google Scholar]
- Kornbrust D. J., Mavis R. D. Microsomal lipid peroxidation. I. Characterization of the role of iron and NADPH. Mol Pharmacol. 1980 May;17(3):400–407. [PubMed] [Google Scholar]
- Lombardi B. Pathogenesis of fatty liver. Fed Proc. 1965 Sep-Oct;24(5):1200–1205. [PubMed] [Google Scholar]
- Marshall W. J., McLean A. E. A requirement for dietary lipids for induction of cytochrome P-450 by phenobarbitone in rat liver microsomal fraction. Biochem J. 1971 May;122(4):569–573. doi: 10.1042/bj1220569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody D. E., James J. L., Smuckler E. A. Cytochrome P-450 lowering effect of alkyl halides, correlation with decrease in arachidonic acid. Biochem Biophys Res Commun. 1980 Nov 28;97(2):673–679. doi: 10.1016/0006-291x(80)90317-4. [DOI] [PubMed] [Google Scholar]
- Moore L., Rodman Davenport G., Landon E. J. Calcium uptake of a rat liver microsomal subcellular fraction in response to in vivo administration of carbon tetrachloride. J Biol Chem. 1976 Feb 25;251(4):1197–1201. [PubMed] [Google Scholar]
- RECKNAGEL R. O., LITTERIA M. Biochemical changes in carbon tetrachloride fatty liver: concentration of carbon tetrachloride in liver and blood. Am J Pathol. 1960 May;36:521–531. [PMC free article] [PubMed] [Google Scholar]
- Rao K. S., Recknagel R. O. Early incorporation of carbon-labeled carbon tetrachloride into rat liver particulate lipids and proteins. Exp Mol Pathol. 1969 Apr;10(2):219–228. doi: 10.1016/0014-4800(69)90041-0. [DOI] [PubMed] [Google Scholar]
- Rao K. S., Recknagel R. O. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol. 1968 Oct;9(2):271–278. doi: 10.1016/0014-4800(68)90041-5. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Quantitative estimation of peroxidative degeneration of rat liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning. Exp Mol Pathol. 1966 Oct;5(5):413–426. doi: 10.1016/0014-4800(66)90023-2. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S. Comparison of early injury to liver endoplasmic reticulum by halomethanes, hexachloroethane, benzene, toluene, bromobenzene, ethionine, thioacetamide and dimethylnitrosamine. Biochem Pharmacol. 1972 Oct 1;21(19):2555–2561. doi: 10.1016/0006-2952(72)90223-7. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S., Moslen M. T. In vivo covalent binding of 14CCl4 metabolites in liver microsomal lipids. Biochem Biophys Res Commun. 1974 Apr 8;57(3):747–750. doi: 10.1016/0006-291x(74)90609-3. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S., Yee A. G. Liver parenchymal cell injury. V. Relationships between patterns of chloromethane-C 14 incorporation into constituents of liver in vivo and cellular injury. Lab Invest. 1967 Apr;16(4):591–603. [PubMed] [Google Scholar]
- Rowe L., Wills E. D. The effect of dietary lipids and vitamin E on lipid peroxide formation, cytochrome P-450 and oxidative demethylation in the endoplasmic reticulum. Biochem Pharmacol. 1976 Jan 15;25(2):175–179. doi: 10.1016/0006-2952(76)90287-2. [DOI] [PubMed] [Google Scholar]
- SMUCKLER E. A., ISERI O. A., BENDITT E. P. An intracellular defect in protein synthesis induced by carbon tetrachloride. J Exp Med. 1962 Jul 1;116:55–72. doi: 10.1084/jem.116.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D., Higginson J. A comparison of ultrastructural changes in rat liver due to chemical carcinogens. Cancer Res. 1968 Sep;28(9):1703–1733. [PubMed] [Google Scholar]
- Willis R. J. Possible role of endogenous toxigenic lipids in the carbon tetrachloride poisoned hepatocyte. Fed Proc. 1980 Nov;39(13):3134–3137. [PubMed] [Google Scholar]
- del Villarruel M., de Toranzo E. G., Castro J. A. CC14 administration to strain A/J mice or rats and the arachidonic acid content of their liver microsomal prospholipids. Res Commun Chem Pathol Pharmacol. 1976 May;14(1):193–196. [PubMed] [Google Scholar]


