Abstract
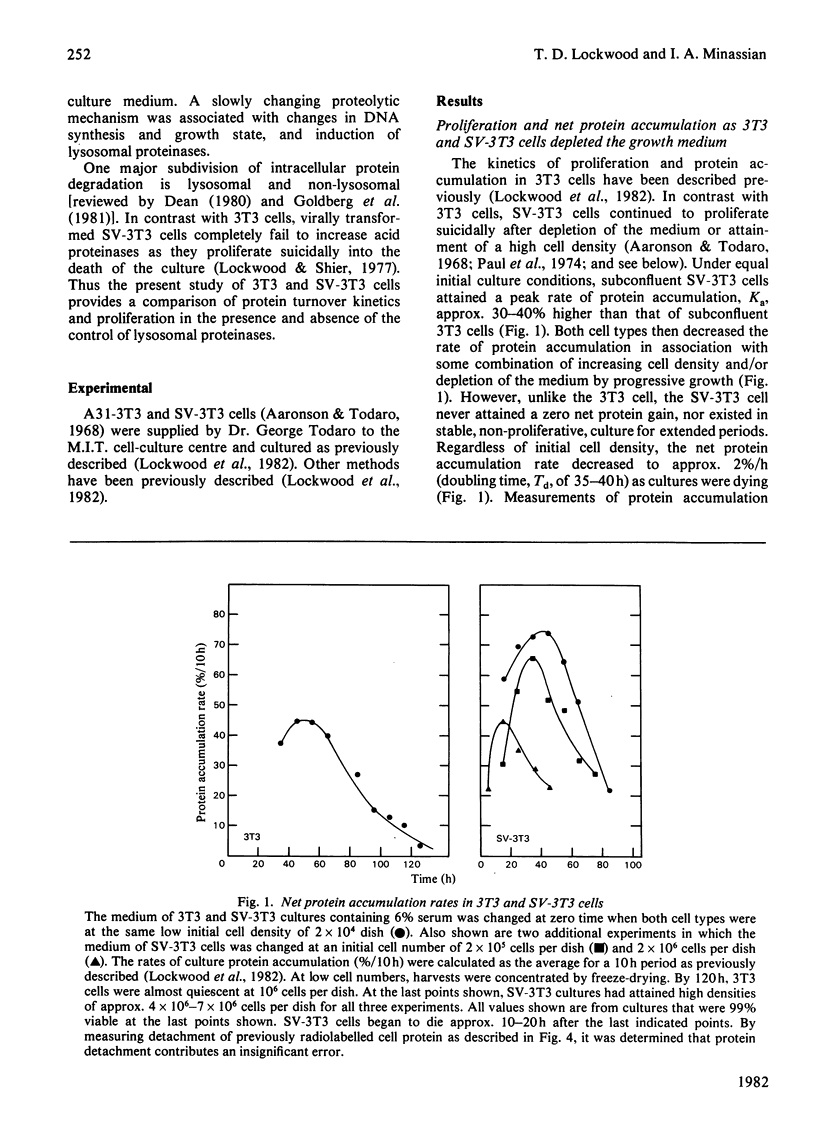
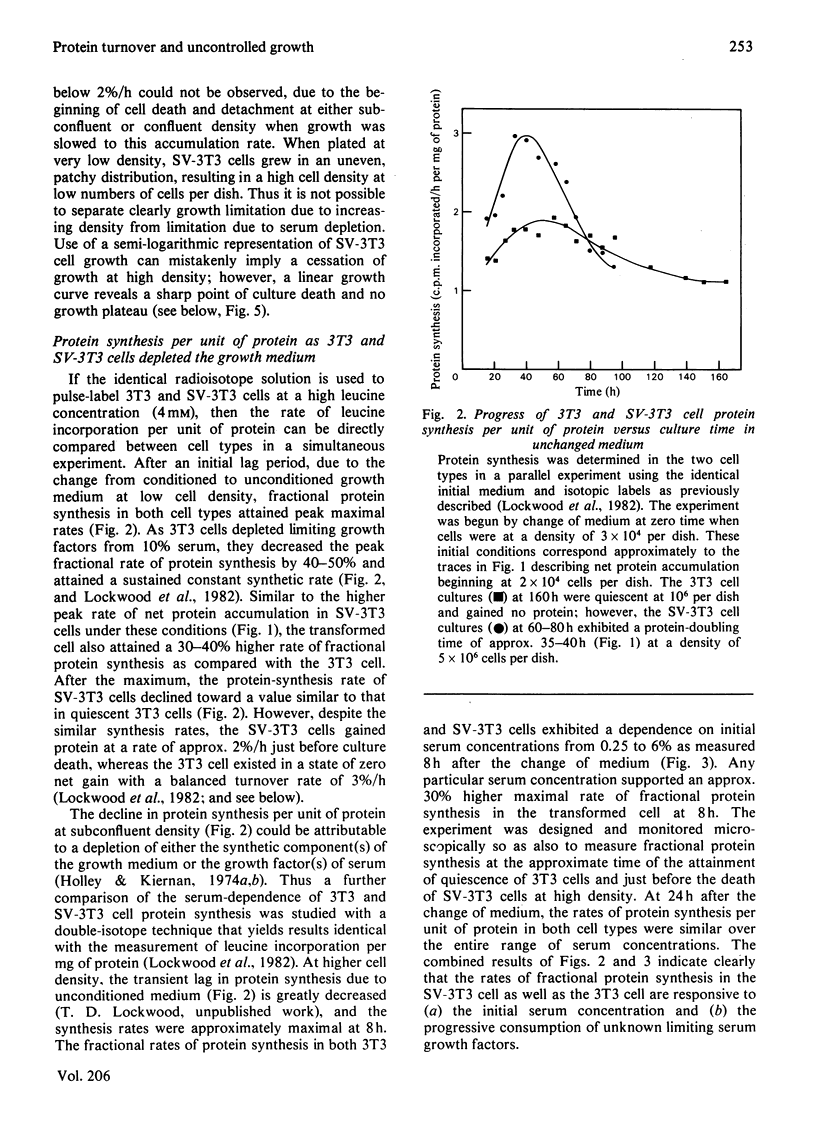
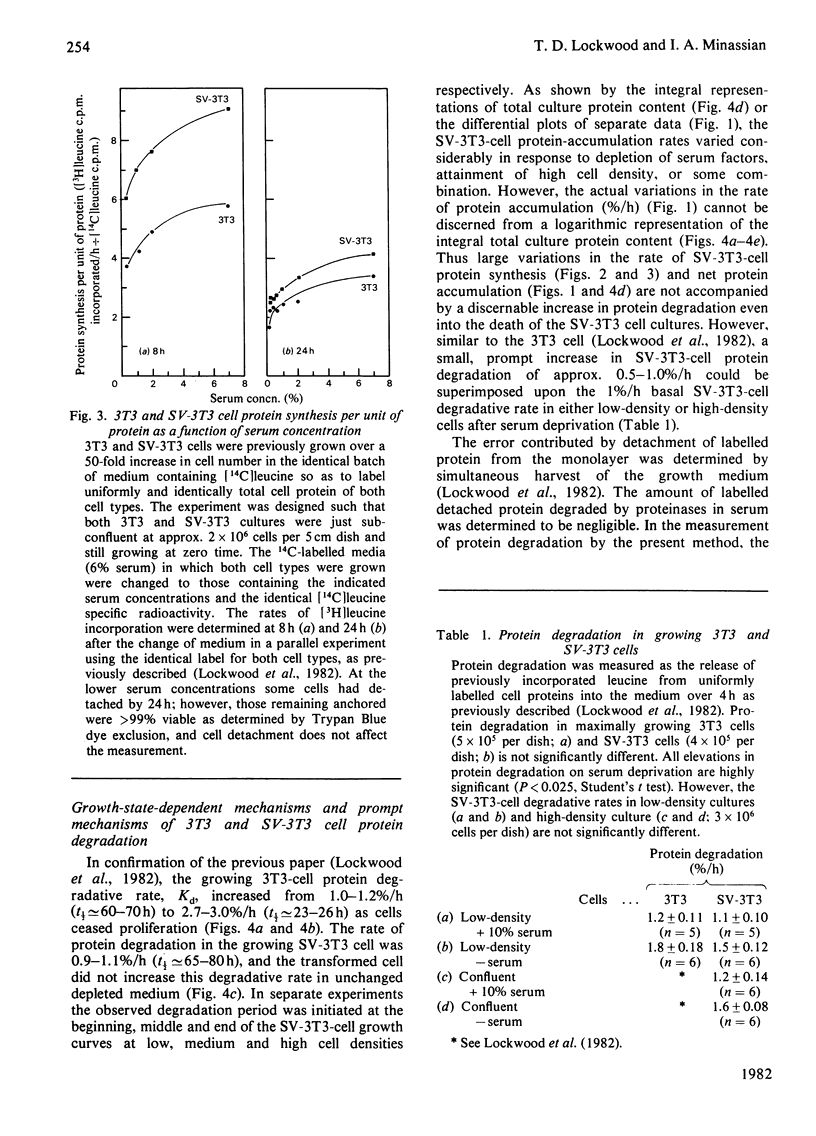
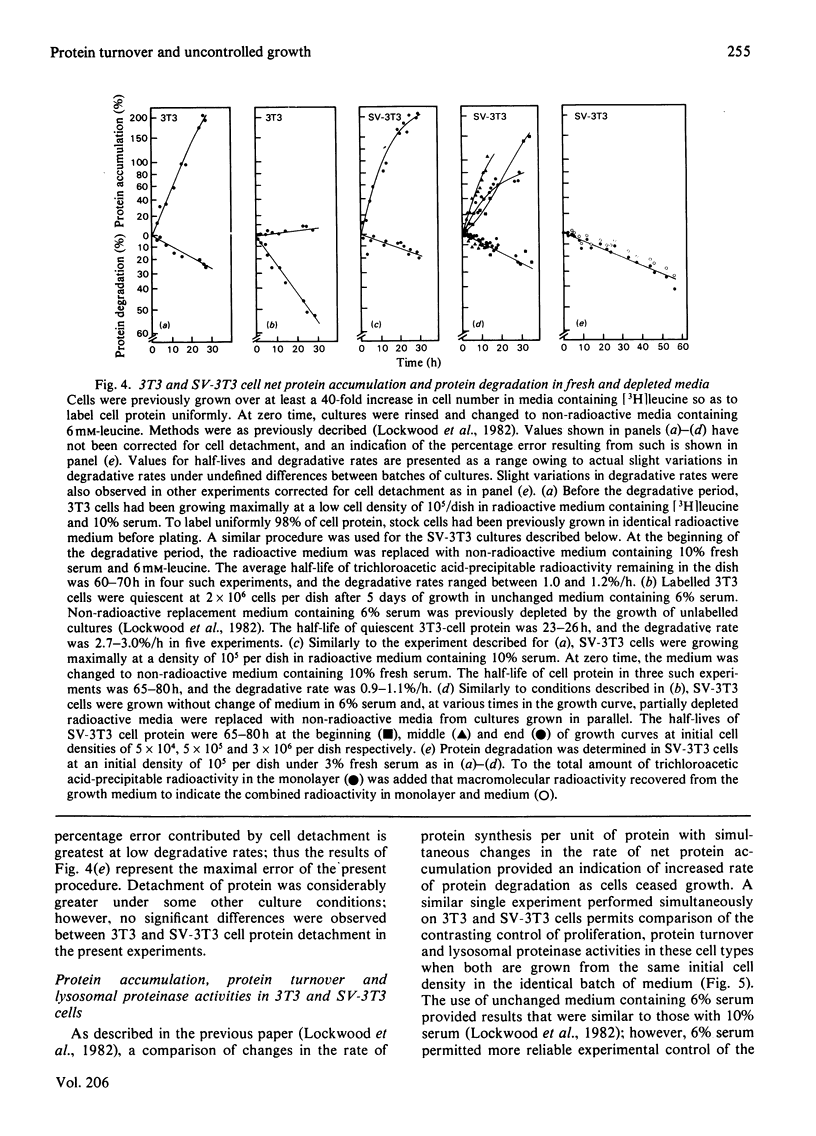
The contrasting control of lysosomal proteinases, protein turnover and proliferation was studied in 3T3 and SV-3T3 (SV-40-virus-transformed 3T3) cells. 1. In 3T3 cells, net protein accumulation proceeded from 5%/h (doubling time, Td=14h) in growing cells to 0%/h as cells became quiescent. SV-3T3 cells never ceased to gain protein, but rather decreased their protein accumulation rate from 6–7%/h (Td=10–12h) to 2%/h (Td=35–40h) just before culture death in unchanged medium. 2. In both cell types the rates of protein synthesis per unit of protein (a) were proportional to the initial serum concentration from 0 to 6%, and (b) declined under progressive depletion of undefined serum growth factors. In depleted growth medium, leucine incorporation per unit of protein in 3T3 and SV-3T3 cells declined to almost equal synthetic rates while the 3T3 cell existed in a steady state of zero net gain, and the SV-3T3 cell continued to gain protein at a rate of 2%/h. 3. Whereas a large fraction of the control of 3T3-cell net protein accumulation can be accounted for by an increase in degradation from 1%/h to 3%/h, the SV-3T3 cell did not exhibit a growth-related increase in degradation appreciably above 1%/h. 4. Thus, by using first-order kinetics, the continued net protein accumulation of the transformed cell can be accounted for by a failure to increase protein degradation, whereas fractional synthesis can be made to decline to a rate similar to that in the quiescent non-transformed cell. 5. Upon acute serum deprivation, both cell types similarly exhibited small rapid increases in proteolysis independent of cell growth state or lysosomal enzyme status. 6. The 3T3 cell increased its lysosomal proteinase activity in conjunction with increase in the growth-state-dependent proteolytic mechanism; however, the SV-3T3 cell failed to increase lysosomal proteinases or the growth-state-dependent proteolytic mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Brocher S. C. Protein synthesis and degradation in growth regulation in rat embryo fibroblasts: role of fast-turnover and slow-turnover protein. J Cell Physiol. 1980 Oct;105(1):51–61. doi: 10.1002/jcp.1041050108. [DOI] [PubMed] [Google Scholar]
- Baxter G. C., Stanners C. P. The effect of protein degradation on cellular growth characteristics. J Cell Physiol. 1978 Aug;96(2):139–145. doi: 10.1002/jcp.1040960202. [DOI] [PubMed] [Google Scholar]
- Bradley M. O. Regulation of protein degradation in normal and transformed human cells. Effects of growth state, medium composition, and viral transformation. J Biol Chem. 1977 Aug 10;252(15):5310–5315. [PubMed] [Google Scholar]
- Dean R. T. Protein degradation in cell cultures: general considerations on mechanisms and regulation. Fed Proc. 1980 Jan;39(1):15–19. [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Gunn J. M., Clark M. G., Knowles S. E., Hopgood M. F., Ballard F. J. Reduced rates of proteolysis in transformed cells. Nature. 1977 Mar 3;266(5597):58–60. doi: 10.1038/266058a0. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hendil K. B. Intracellular protein degradation in growing, in density-inhibited, and in serum-restricted fibroblast cultures. J Cell Physiol. 1977 Sep;92(3):353–364. doi: 10.1002/jcp.1040920304. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer H., Heinrich P. C. Control of proteolysis. Annu Rev Biochem. 1980;49:63–91. doi: 10.1146/annurev.bi.49.070180.000431. [DOI] [PubMed] [Google Scholar]
- Lockwood T. D., Minassian I. A., Roux L. Protein turnover and proliferation. Turnover kinetics associated with the elevation of 3T3-cell acid-proteinase activity and cessation of net protein gain. Biochem J. 1982 Aug 15;206(2):239–249. doi: 10.1042/bj2060239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood T. D., Shier W. T. Regulation of acid proteases during growth, quiescence and starvation in normal and transformed cells. Nature. 1977 May 19;267(5608):252–254. doi: 10.1038/267252a0. [DOI] [PubMed] [Google Scholar]
- Olsen I., Dean M. F., Harris G., Muir H. Direct transfer of a lysosomal enzyme from lymphoid cells to deficient fibroblasts. Nature. 1981 May 21;291(5812):244–247. doi: 10.1038/291244a0. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Paul D., Henahan M., Walter S. Changes in growth control and growth requirements associated with neoplastic transformation in vitro. J Natl Cancer Inst. 1974 Nov;53(5):1499–1503. doi: 10.1093/jnci/53.5.1499. [DOI] [PubMed] [Google Scholar]
- Poole B., Wibo M. Protein degradation in cultured cells. The effect of fresh medium, fluoride, and iodoacetate on the digestion of cellular protein of rat fibroblasts. J Biol Chem. 1973 Sep 10;248(17):6221–6226. [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R. The mannose 6-phosphate receptor of Chinese hamster ovary cells. Compartmentalization of acid hydrolases in mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10623–10627. [PubMed] [Google Scholar]
- Warburton M. J., Poole B. Effect of medium composition on protein degradation and DNA synthesis in rat embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2427–2431. doi: 10.1073/pnas.74.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]


