Abstract
1. The increase in the haem saturation of rat liver tryptophan pyrrolase caused by tryptophan administration was previously shown to be associated with a decrease in 5-aminolaevulinate synthase activity. 2. It is now shown that similar reciprocal effects are caused by palmitate and salicylate, both of which increase tryptophan availability to the liver by direct displacement of the serum-protein-bound amino acid. 3. The reciprocal effects on the former two parameters caused by endotoxin and morphine are associated with an increase in liver tryptophan concentration produced by a lipolysis-dependent, non-esterified fatty acid-mediated, displacement of the serum-protein-bound amino acid. 4. All these changes and those caused by another lipolytic agent, theophylline, are prevented by the β-adrenoceptor-blocking agent propranolol and by the opiate-receptor antagonist naloxone, whose anti-lipolytic nature is demonstrated. 5. High correlation coefficients have been obtained for one or more pairs of the following parameters: serum non-esterified fatty acid concentration, free serum tryptophan concentration, liver tryptophan concentration, liver 5-aminolaevulinate synthase activity, liver holo-(tryptophan pyrrolase) activity and the haem saturation of liver tryptophan pyrrolase. 6. It is suggested that liver tryptophan concentration may play an important role in the regulation of 5-aminolaevulinate synthase synthesis, and that the latter may be subject to control by changes in lipid metabolism and may be influenced by pharmacological agents that affect tryptophan disposition. 7. Preliminary evidence suggests that tryptophan may be bound in the liver and that such a possible binding may control its availability for its hepatic functions.
Full text
PDF







Selected References
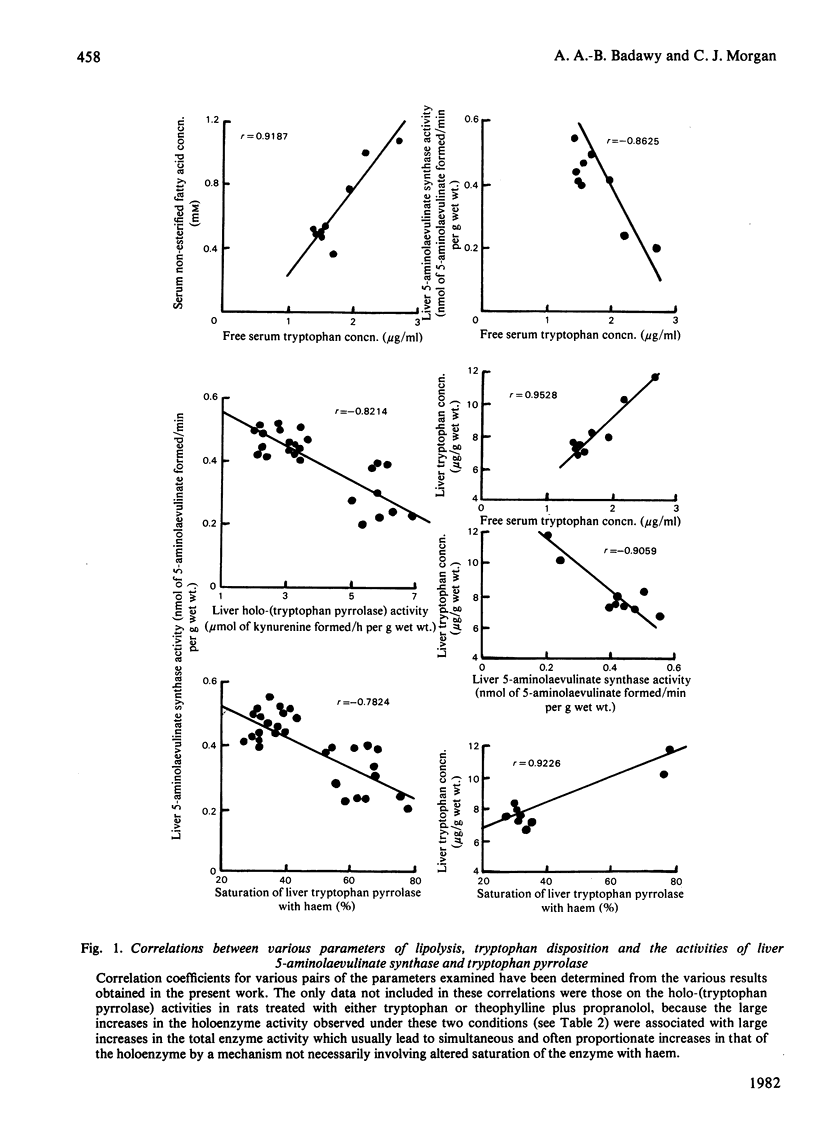
These references are in PubMed. This may not be the complete list of references from this article.
- Badawy A. A., Evans M. Animal liver tryptophan pyrrolases: Absence of apoenzyme and of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem J. 1976 Jul 15;158(1):79–88. doi: 10.1042/bj1580079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: Experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975 Sep;150(3):511–520. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of chemical porphyrogens and drugs on the activity of rat liver tryptophan pyrrolase. Biochem J. 1973 Dec;136(4):885–892. doi: 10.1042/bj1360885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of ethanol on tryptophan pyrrolase activity and their comparison with those of phenobarbitone and morphine. Adv Exp Med Biol. 1975;59:229–251. doi: 10.1007/978-1-4757-0632-1_15. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The mechanism of inhibition of rat liver tryptophan pyrrolase activity by 4-hydroxypyrazolo(3,4-d)pyrimidine (Allopurinol). Biochem J. 1973 Jul;133(3):585–591. doi: 10.1042/bj1330585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The role of free serum tryptophan in the biphasic effect of acute ethanol administration on the concentrations of rat brain tryptophan, 5-hydroxytryptamine and 5-hydroxyindol-3-ylacetic acid. Biochem J. 1976 Nov 15;160(2):315–324. doi: 10.1042/bj1600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. Heme utilization by rat liver tryptophan pyrrolase as a screening test for exacerbation of hepatic porphyrias by drugs. J Pharmacol Methods. 1981 Sep;6(2):77–85. doi: 10.1016/0160-5402(81)90030-9. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Morgan C. J. Tryptophan pyrrolase in haem regulation. The relationship between the depletion of rat liver tryptophan pyrrolase haem and the enhancement of 5-aminolaevulinate synthase activity by 2-allyl-2-isopropylacetamide. Biochem J. 1980 Mar 15;186(3):763–772. doi: 10.1042/bj1860763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Smith M. J. Changes in liver tryptophan and tryptophan pyrrolase activity after administration of salicylate and tryptophan to the rat. Biochem Pharmacol. 1972 Jan;21(1):97–101. doi: 10.1016/0006-2952(72)90254-7. [DOI] [PubMed] [Google Scholar]
- Badawy A. A. The effects of acetate, metal cations, phenobarbitone, porphyrogens and substrates of glycine acyltransferase on the utilization of haem by rat liver apo-(tryptophan pyrrolase). Biochem J. 1977 May 15;164(2):431–438. doi: 10.1042/bj1640431. [DOI] [PMC free article] [PubMed] [Google Scholar]
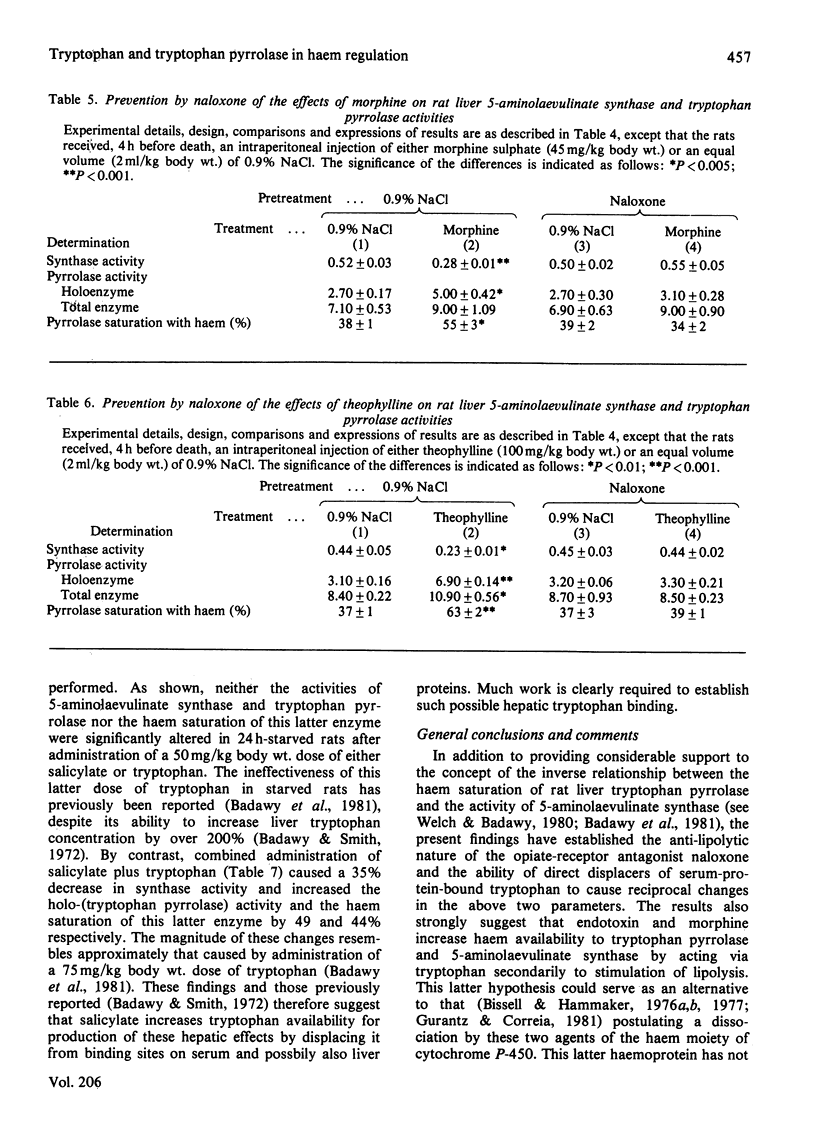
- Badawy A. A., Welch A. N., Morgan C. J. Tryptophan pyrrolase in haem regulation. The mechanism of the opposite effects of tryptophan on rat liver 5-aminolaevulinate synthase activity and the haem saturation of tryptophan pyrrolase. Biochem J. 1981 Aug 15;198(2):309–314. doi: 10.1042/bj1980309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome P-450 heme and the regulation of hepatic heme oxygenase activity. Arch Biochem Biophys. 1976 Sep;176(1):91–102. doi: 10.1016/0003-9861(76)90144-2. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome p-450 heme and the regulation of delta-aminolevulinic acid synthetase in the liver. Arch Biochem Biophys. 1976 Sep;176(1):103–112. doi: 10.1016/0003-9861(76)90145-4. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Effect of endotoxin on tryptophan pyrrolase and delta-aminolaevulinate synthase: evidence for an endogenous regulatory haem fraction in rat liver. Biochem J. 1977 Aug 15;166(2):301–304. doi: 10.1042/bj1660301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum I., Schoenfeld N., Atsmon A. The effect of DL-propranolol on delta-aminolevulinic acid synthetase activity and urinary excretion of porphyrins in allylisopropylacetamide-induced experimental porphyria. Biochim Biophys Acta. 1973 Sep 14;320(2):242–248. doi: 10.1016/0304-4165(73)90304-8. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- Curzon G., Friedel J., Katamaneni B. D., Greenwood M. H., Lader M. H. Unesterified fatty acids and the binding of tryptophan in human plasma. Clin Sci Mol Med. 1974 Nov;47(5):415–424. doi: 10.1042/cs0470415. [DOI] [PubMed] [Google Scholar]
- Curzon G., Friedel J., Knott P. J. The effect of fatty acids on the binding of tryptophan to plasma protein. Nature. 1973 Mar 16;242(5394):198–200. doi: 10.1038/242198a0. [DOI] [PubMed] [Google Scholar]
- Druyan R., Kelly A. The effect of exogenous -aminolaevulinate on rat liver haem and cytochromes. Biochem J. 1972 Oct;129(5):1095–1099. doi: 10.1042/bj1291095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurantz D., Correia M. A. Morphine-mediated effects on rat hepatic heme and cytochrome P-450 in vivo: antagonism by naloxone in the liver. Biochem Pharmacol. 1981 Jun 15;30(12):1529–1536. doi: 10.1016/0006-2952(81)90377-4. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. Effects of administration of glucose, cyclic AMP, and some hormones related to glucose metabolism. Arch Biochem Biophys. 1974 Sep;164(1):293–304. doi: 10.1016/0003-9861(74)90034-4. [DOI] [PubMed] [Google Scholar]
- McArthur J. N., Dawkins P. D. The effect of sodium salicylate on the binding of L-tryptophan to serum proteins. J Pharm Pharmacol. 1969 Nov;21(11):744–750. doi: 10.1111/j.2042-7158.1969.tb08163.x. [DOI] [PubMed] [Google Scholar]
- Mikac-Dević D., Stanković H., Bosković K. A method for determination of free fatty acids in serum. Clin Chim Acta. 1973 Apr 19;45(1):55–59. doi: 10.1016/0009-8981(73)90144-7. [DOI] [PubMed] [Google Scholar]
- Morgan C. J., Badawy A. A. Tryptophan pyrrolase in haem regulation. The mechanism of the permissive effect of cortisol on the enhancement of 5-aminolaevulinate synthase activity by 2-allyl-2-isopropylacetamide in the adrenalectomized-rat liver. Biochem J. 1980 Mar 15;186(3):993–996. doi: 10.1042/bj1860993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B. A., Yoda B., Israels L. G. Cyclic oscillations in rat hepatic heme oxygenase and delta-aminolevulinic acid synthetase following intravenous heme administration. Arch Biochem Biophys. 1976 Mar;173(1):11–17. doi: 10.1016/0003-9861(76)90228-9. [DOI] [PubMed] [Google Scholar]
- Welch A. N., Badawy A. A. Tryptophan pyrrolase in haem regulation. Experiments with administered haematin and the relationship between the haem saturation of tryptophan pyrrolase and the activity of 5-aminolaevulinate synthase in rat liver. Biochem J. 1980 Nov 15;192(2):403–410. doi: 10.1042/bj1920403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterberg L., Yuwiler A., Geller E. Tryptophan oxygenase changes following delta-aminolevulinic acid administration in the rat. Life Sci. 1969 Oct 1;8(19):1047–1049. doi: 10.1016/0024-3205(69)90156-8. [DOI] [PubMed] [Google Scholar]
- Wong S. C., Yeung Y. G., Yeung D. Acute and chronic effects of morphine on lipolysis in rat epididymal fat pads. Biochem Pharmacol. 1977 Jan 15;26(2):143–147. doi: 10.1016/0006-2952(77)90387-2. [DOI] [PubMed] [Google Scholar]


