Abstract
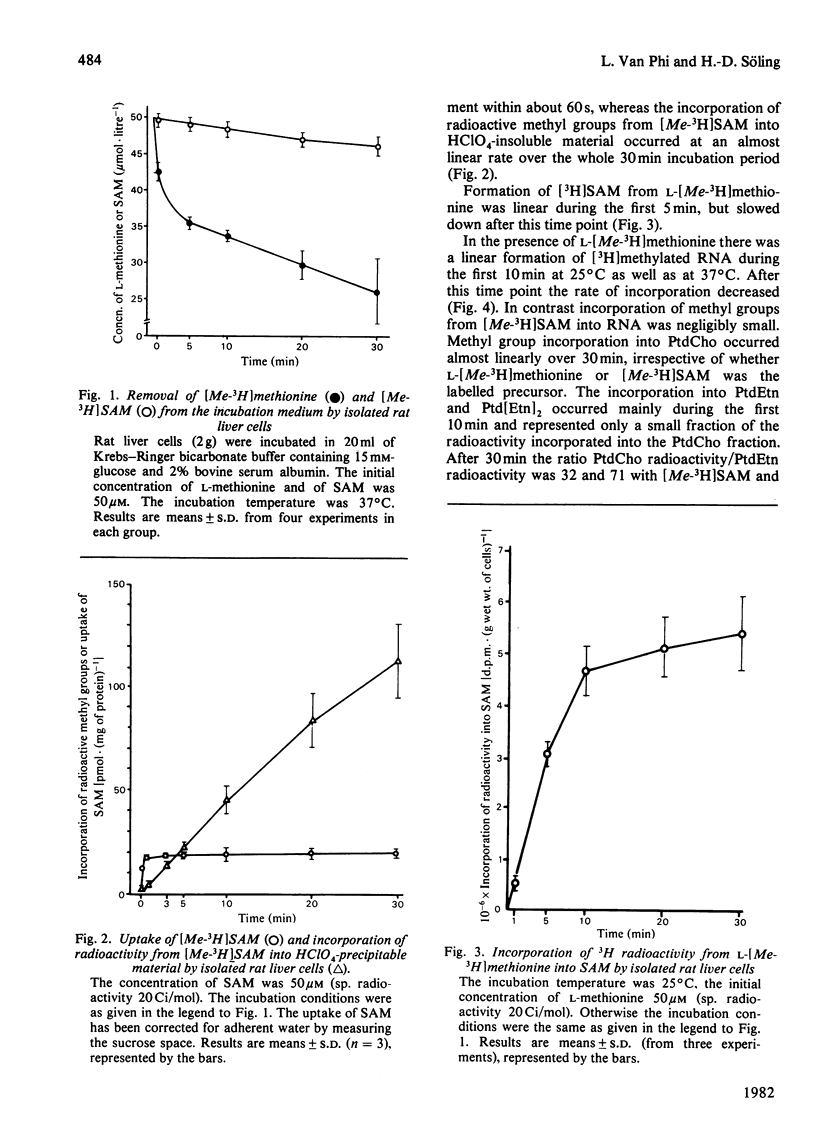
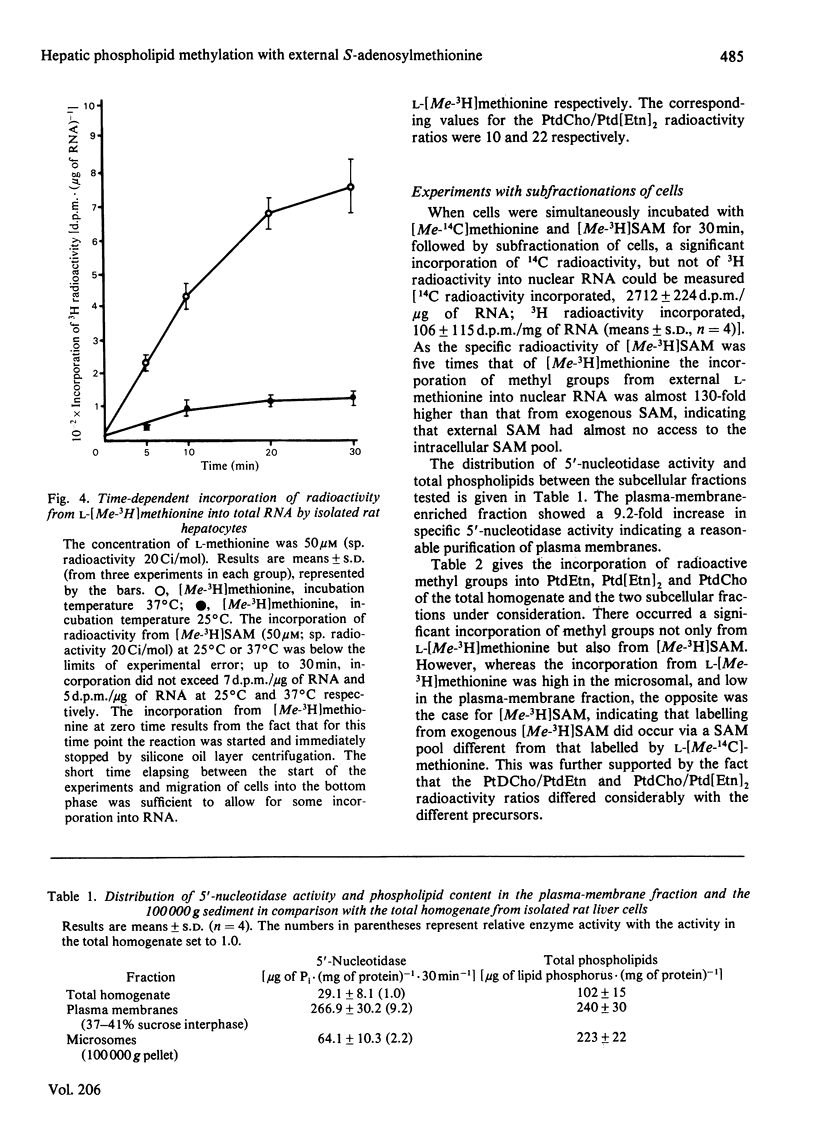
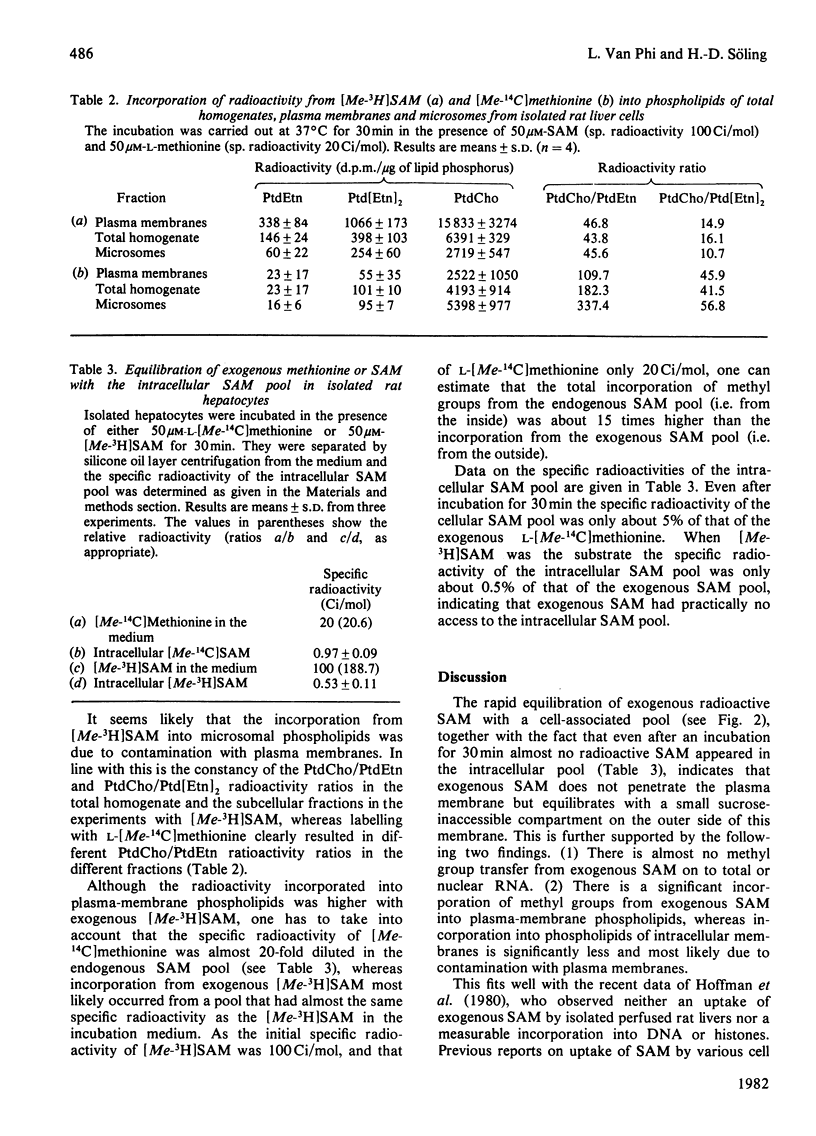
At external concentration of 50 microM, L-methionine was rapidly taken up by hepatocytes, whereas almost no S-adenosylmethionine (SAM) was removed from the incubation medium. SAM did not enter the intracellular water space but equilibrated with a very small pool, which was most likely to be situated on the external side of the plasma membrane. Methyl groups from external L-methionine, but not from external SAM, were incorporated into total and nuclear RNA. A significant incorporation of methyl groups into phospholipids occurred not only with methionine but also with SAM. After subfractionation of hepatocytes it became evident that methyl groups from SAM were mainly incorporated into plasma-membrane phospholipids, and that phospholipid methylation in other cellular compartments resulted from contamination with plasma membrane. The pattern of methylation of the various phospholipid species with SAM as precursor was different from that obtained with L-methionine. In contrast with external L-methionine, external SAM did not enter the intracellular SAM pool. According to these results a transport system for SAM does not exist in rat hepatocytes, although methyl groups from external SAM can be incorporated into plasma-membrane phospholipids from the outside.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling P. R., Le Page R. N. A rapid high yield method for the preparation of rat liver cell plasma membranes. Biochim Biophys Acta. 1973 Aug 9;318(1):33–40. doi: 10.1016/0005-2736(73)90333-7. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Hoffman D. R., Marion D. W., Cornatzer W. E., Duerre J. A. S-Adenosylmethionine and S-adenosylhomocystein metabolism in isolated rat liver. Effects of L-methionine, L-homocystein, and adenosine. J Biol Chem. 1980 Nov 25;255(22):10822–10827. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Spence K. D. Transport of S-adenosylmethionine in Saccharomyces cerevisiae. J Bacteriol. 1972 Feb;109(2):499–504. doi: 10.1128/jb.109.2.499-504.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Schlenk F. Active transport of exogenous S-adenosylmethionine and related compounds into cells and vacuoles of Saccharomyces cerevisiae. J Bacteriol. 1974 Oct;120(1):482–487. doi: 10.1128/jb.120.1.482-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Falvey A. K., Anderson W. F. Preparation of globin messenger RNA. Methods Enzymol. 1974;30:621–630. doi: 10.1016/0076-6879(74)30060-2. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem. 1968 Oct 17;6(1):66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- STEKOL J. A., ANDERSON E. I., WEISS S. S-Adenosyl-L-methionine in the synthesis of choline, creatine, and cysteine in vivo and in vitro. J Biol Chem. 1958 Aug;233(2):425–429. [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F. S-adenosylmethionine in the vacuole of Candida utilis. J Bacteriol. 1960 Jun;79:841–848. doi: 10.1128/jb.79.6.841-848.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence K. D. Mutation of Saccharomyces cerevisiae preventing uptake of S-adenosylmethionine. J Bacteriol. 1971 May;106(2):325–330. doi: 10.1128/jb.106.2.325-330.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramentinoli G., Gualano M., Ideo G. Protective role of S-adenosyl-L-methionine on liver injury induced by D-galactosamine in rats. Biochem Pharmacol. 1978 May 15;27(10):1431–1433. doi: 10.1016/0006-2952(78)90097-7. [DOI] [PubMed] [Google Scholar]
- Stramentinoli G., Pezzoli C., Kienle M. G. Uptake of S-adenosyl-L-methionine by rabbit erythrocytes. Biochem Pharmacol. 1978 May 15;27(10):1427–1430. doi: 10.1016/0006-2952(78)90096-5. [DOI] [PubMed] [Google Scholar]
- Zappia V., Galletti P., Porcelli M., Ruggiero G., Andreana A. Uptake of adenosylmethionine and related sulfur compounds by isolated rat liver. FEBS Lett. 1978 Jun 15;90(2):331–335. doi: 10.1016/0014-5793(78)80398-6. [DOI] [PubMed] [Google Scholar]


