ABSTRACT
Background
Premature ventricular contractions (PVCs) are a frequent electrocardiographic finding in routine medical practice, and 16% of the patients with idiopathic PVCs may have underlying heart disease.
Objective
We analyzed the correlation between the morphology of the PVCs and the myocardial scarring identified by cardiac magnetic resonance (CMR), together with the impact of late gadolinium enhancement (LGE) on the need for ablation.
Methods
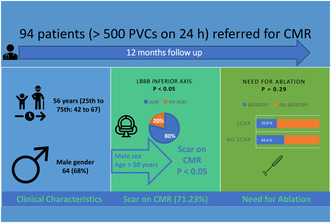
Ninety‐four patients (median age 56 years) with frequent PVCs (> 500 PVCs on 24 h) and a structurally normal heart were referred for comprehensive CMR. The patients were followed for 12 months. Patients were referred for ablation if they were symptomatic in the context of frequent PVCs.
Results
The prevalence of LGE identified by CMR was higher among males (OR 5.506 (2.092–14.49) p < 0.05), with an age ≥ 50 years (OR 1.047 (1.015–1.08), p < 0.05) and a higher PVC burden (OR 1.922 (1.723–1.976), p < 0.05). Additionally, patients with PVCs with a LBBB inferior axis had four times higher risk of exhibiting LGE (OR 4.09 (1.584–10.565), p < 0.05). In multivariate analysis, age (OR 1.059 (1.019–1.099), p < 0.05) and a LBBB inferior axis (OR 4.605 (1.472–14.404), p < 0.05) were independently associated with the presence of LGE.
Conclusion
Patients with PVCs and apparently, structurally normal heart present myocardial scarring identified by CMR in 71.23% of cases. PVCs with LBBB inferior axis pattern, age ≥ 50 years and male sex are associated with the presence of LGE on CMR. In multivariate analysis, age and LBBB inferior axis were independently correlated with the presence of LGE.
Keywords: ablation, cardiac magnetic resonance, late gadolinium enhancement, premature ventricular contractions
Ninety‐four patients with idiopathic PVCs were referred for CMR. 71.23% of the patients had scar detected by CMR. Male sex, an age > 50 years old and a LBBB inferior axis were independent predictors of scar. The presence of scar didn't correlate with the need for ablation.
Abbreviations
- AADs
anti‐arrhythmic drugs
- BMI
body mass index
- CMR
cardiac magnetic resonance
- ECG
electrocardiography
- EDV
end‐diastolic volume
- EF
- ESV
end‐systolic volume
- IQR
interquartile range
- IVS
interventricular septum
- LAVI
left atrial volume index
- LBBB
left bundle branch block
- LGE
late gadolinium enhancement
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- LVMI
left ventricular mass index
- LVOT
left ventricular outflow tract
- PVCs
premature ventricular contractions
- RBBB
right bundle branch block
- RV FAC
right ventricular fractional area change
- RVOT
right ventricular outflow tract
- SCD
sudden cardiac death
- SD
standard deviation
- TTE
transthoracic echocardiography
- VAs
ventricular arrhythmias
- VF
ventricular fibrillation
- VT
ventricular tachycardia
1. Introduction
Premature ventricular contractions (PVCs) are a frequent electrocardiographic (ECG) finding in routine medical practice. Most of the patients with PVCs present with palpitations, but about a third of them remain asymptomatic (Sorgente et al. 2022). Older age, taller stature, male sex, and certain controllable factors like smoking, higher body mass index (BMI), physical inactivity, and stimulant use are associated with a higher likelihood of experiencing PVCs (Nguyen et al. 2017; Marcus 2020).
PVCs in patients with a structurally normal heart at routine diagnosis workup are considered idiopathic. Among individuals ≥ 18 years, the incidence of idiopathic PVCs is reported to be around 51.9 per 100,000, with an increasing incidence with aging (Sirichand et al. 2017). Idiopathic PVCs originate, in almost 70% of cases, from the right and left ventricular outflow tract, and they account for 10% of all ventricular arrhythmias (Sorgente et al. 2022) referred for catheter ablation (Maury et al. 2015). The remaining 30% of PVCs originate from non‐outflow tract structures including the left and right papillary muscles (5%–15%), mitral annulus (5%), tricuspid annulus (8%–10%), left bundle branch fascicles (10%), cardiac crux and moderator band (Muser et al. 2021).
Despite a normal workup, patients with idiopathic PVCs may have hidden underlying heart disease in up to 16%, which is proven to be associated with worse clinical outcomes, highlighting the importance of distinguishing between idiopathic and non‐idiopathic PVCs (You and Liu 2019; Muser et al. 2020). To rule out structural cardiac disease the initial routine evaluation includes ECG, transthoracic echocardiography (TTE), and evaluation of the coronary arteries (either by computed tomography coronary angiography or invasive coronary angiography).
In the last decades, cardiac magnetic resonance (CMR) has been used as an imaging technique being able to identify myocardial abnormalities in patients with PVCs and apparently structurally normal heart after routine evaluation (Muser et al. 2020). The importance of CMR imaging lies in its ability to precisely evaluate the function of both the left ventricle (LV) and right ventricle (RV). Furthermore, CMR can detect characteristics of myocardial tissue, including focal fatty infiltration, tissue edema, and areas of necrosis or fibrosis by late gadolinium enhancement (LGE). Focal myocardial abnormalities may indicate localized inflammatory processes, such as previous myocarditis, or may indicate the initial stages of a cardiomyopathy and might be identified in up to half of patients with frequent PVCs and normal ECG and echocardiographic results (Sassone et al. 2019; Muser et al. 2018).
In this context, we performed a cross‐sectional prospective observational study, including a cohort of patients with idiopathic PVCs, in order to determine the main predictors of the presence of LGE detected by CMR.
2. Materials and Methods
2.1. Study Population
A prevalent cross‐sectional cohort approach was used and patients with frequent PVCs (> 500/24 h) referred by their cardiologist to our tertiary adult Arrhythmia Center were assessed for eligibility for inclusion. We included patients who had > 500 PVCs/24 h, normal 12‐lead surface ECG, and 2D transthoracic echocardiography, with absence of significant coronary artery disease demonstrated by computed tomography coronary angiography or invasive coronary angiography. The main exclusion criteria were any pre‐existing structural or ischemic heart disease.
2.2. Study Design
This was a cross‐sectional cohort study which involved the screening of 125 patients with PVCs referred for an electrophysiology consultation by their cardiologist between March 2021 and November 2022. This study was conducted in accordance with the principles embodied in the Declaration of Helsinki and in accordance with local statutory requirements. After obtaining the informed consent, every patient underwent clinical examination, ECG, Holter monitoring, and echocardiography. After analyzing the results, 94 patients with frequent PVCs (> 500 PVCs on 24 h) and a structurally normal heart were included (as defined by the inclusion criteria). All these patients were then referred for CMR (Figure 1). Afterward, patients were followed up for 12 months, with echocardiography and 24 h Holter monitoring every 3 months, in order to determine the need for ablation.
FIGURE 1.
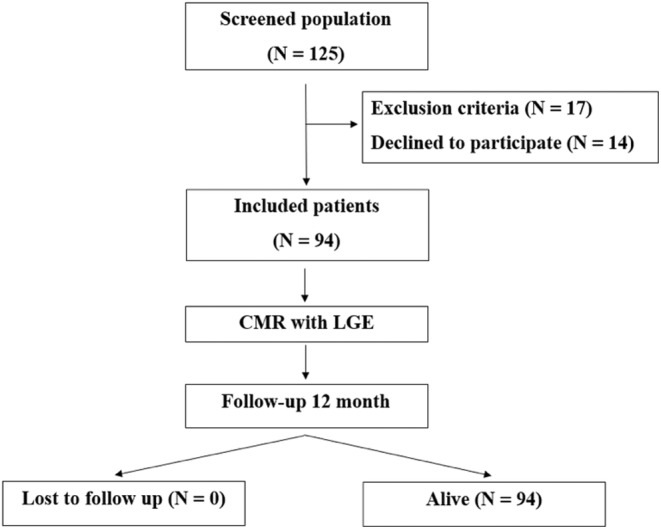
Study design. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
The primary outcome of this study was the correlation between the morphology of the PVCs on the surface ECG and the myocardial scarring identified by CMR with LGE imaging. The secondary outcome was the impact of LGE presence on the need for ablation in patients idiopathic PVCs.
2.3. Clinical Data, ECG, Holter Monitoring, Echocardiography, CMR
All basic clinical data was collected at the time of study enrollment and included information about smoking, family history of sudden cardiac death, history of unexplained syncope, symptoms related to arrhythmia, prescription of antiarrhythmic drugs, history of hypertension, diabetes, dyslipidemia. In addition, body weight, height, and body surface area were determined (to calculate the body mass index).
The baseline 12 leads ECGs were performed using the Philips PageWriter TC50 electrocardiograph and analyzed by experienced operators (at least 3 years of experience). Various ECG parameters were used: native QRS axis, presence of native QRS fragmentation, native QRS width, PVC QRS width, PVC QRS axis, PVC coupling interval. Multifocal PVCs were defined as more than two different QRS morphologies (Haruta et al. 2016).
At enrollment, all patients were monitored with a 12‐channel Holter ECG for 24 h. The monitoring was performed using the Cardiospy EC‐12H and interpreted by experienced operators (at least 3 years of experience). The number of PVCs and the PVC burden was quantified individually for each patient. Additionally, multifocal PVCs were defined as more than two different QRS morphologies on the Holter monitoring (Ephrem et al. 2013).
Transthoracic echocardiography (TTE) was performed at baseline by the same observer with at least 3 years of experience using the General Electric Vivid S70N Ultra Edition, according to the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. All patients benefited from a comprehensive examination, including the cardiac anatomy (thickness of the interventricular septum (IVS) and posterior wall at end‐diastole, left ventricular mass index (LVMI), left ventricular (LV) end‐diastolic dimension (LVEDD), left atrial volume index (LAVI)), and function (left ventricular ejection fraction (EF) using Simpson's biplane method, right ventricular (RV) fractional area change (FAC)) (Lang et al. 2015).
All patients with normal TTE (no evidence of structural heart disease) underwent comprehensive CMR on a 1.5‐T platform (Siemens Magnetom Altea 1.5 T) using a standardized protocol. The study protocol included the following native acquisitions: (i) long‐ and short‐axis cine sequences, (ii) tissue mapping (native T1, native T2, STIR). Ten minutes after intravenous gadolinium contrast administration (0.1 mmol/kg Gadovist, Bayer Healthcare) LGE images were obtained in three long‐axis views and a complete short‐axis stack, with additional dark‐blood LGE images in cases where necessary if the presence of a subendocardial scar was uncertain. Image processing and analysis were conducted by two experienced operators using the CVI 42 software (Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Volumes and mass were obtained by delineation of endocardial and epicardial borders in short‐axis (ventricles) and long‐axis (atria) cine images at the end of systole and diastole, excluding trabeculations. All the measurements were indexed to body surface area. LGE was defined by the presence of visual enhancement in at least one segment, in either two perpendicular planes or in both bright and black blood images. Subendocardial enhancement with a coronary distribution was defined as ischemic LGE. All other patterns of fibrosis were categorized as non‐ischemic, with the exception of fibrosis at the right ventricular insertion points of the interventricular septum that was not reported. The LGE percentage was calculated for each participant as the sum of hyperenhanced regions with +5 SD signal intensity above the normal remote myocardium, divided by the total LV myocardial mass, expressed as the percentage of the enhanced myocardial mass (Kramer et al. 2020).
2.4. Statistical Analysis
Statistical analysis was performed using SPSS Statistics 25 software (IBM, Armonk, NY, USA). Non‐normally distributed variables were expressed as median with interquartile range (IQR), and normally distributed variables as mean ± standard deviation (SD). For categorical variables, chi‐square test or Fisher's exact test when appropriate was used. An independent t‐test was used for comparing the means of two independent groups of continuous variables. In order to determine the correlations between the variables, Pearson's correlation coefficient was used. Stepwise multivariate regression analysis including all univariate associates (p < 0.05) was used to assess the independent associations between variables.
3. Results
3.1. Study Population
Baseline demographic and clinical characteristics are depicted in Table 1. After screening 125 patients, a total of 94 patients with idiopathic PVCs were included in this study. The median age was 56 years (25th to 75th percentile: 42 to 67), with 64 men (68%) and 30 females (32%) included. Family history of sudden cardiac death was found in 2 (2.13%) patients; 6 (6.38%) patients had history of unexplained syncope. Of all symptomatic patients, 74 (78.72%) had palpitations, 22 (23.4%) were dyspneic, 8 (8.51%) had chest pain, whereas 18 (19.15%) were asymptomatic. The most common anti‐arrhythmic drugs used were Beta‐blockers (28 (29.78%) patients), followed by Class IC anti‐arrhythmic drugs (AADs) (25 (26.59%) patients). The mean BMI was 28.55 ± 4.5, 19 (20.21%) patients were hypertensive, 9 (9.57%) had diabetes, 18 (19.15%) had dyslipidemia, and 5 (5.32%) patients were smokers.
TABLE 1.
Baseline characteristics of the study population.
| Age, years | 56 (42–67) |
| Male Sex | 64 (68%) |
| Family history of sudden cardiac death | 2 (2.13%) |
| History of unexplained syncope | 6 (6.38%) |
| Symptoms | |
| Palpitations | 74 (78.72%) |
| Dyspnea | 22 (23.4%) |
| Asymptomatic | 18 (19.15%) |
| Chest pain | 8 (8.51%) |
| Medical therapy | |
| Class IC AADs | 25 (26.59%) |
| Beta‐blockers | 28 (29.78%) |
| Sotalol | 6 (6.38%) |
| Amiodarone | 6 (6.38%) |
| Other characteristics | |
| Hypertension | 19 (20.21%) |
| Diabetes | 9 (9.57%) |
| Dyslipidemia | 18 (19.15%) |
| Smoking | 5 (5.32%) |
| BMI, kg/m2 | 28.55 ± 4.5 |
Note: Values are median (25th to 75th percentile), mean ± SD or n (%).
Abbreviations: AADs, anti‐arrhythmic drugs; BMI, body mass index.
All patients had biventricular volumes and function within normal 25th to 75th percentile on TTE. Mean LV ejection fraction (EF) was 58% ± 7%; mean LV end‐diastolic volume (EDV) index was 47 ± 14 mL/m2 mean right ventricular fractional area change (RV FAC) was 47 ± 1 2%; there were no significant valvopathies and structural heart disease (Table 2).
TABLE 2.
TTE findings.
| Left ventricle | |
| EDV index, mL/m2 | 47 ± 14 |
| EF, % | 58 ± 7 |
| IVS thickness, mm | 7.9 ± 1.8 |
| LVMI, g/m2 | 67 ± 15 |
| Right ventricle | |
| RV FAC, % | 47 ± 12 |
| Left atrium | |
| LAVI, mL/m2 | 55 ± 10 |
Note: Values are mean ± SD and n (%).
Abbreviations: EDV, end‐diastolic volume; EF, ejection fraction; IVS, interventricular septum; LAVI, left atrial volume index; LVMI, left ventricular mass index; RV FAC, right ventricular fractional area change; TTE, transthoracic echocardiography.
3.2. Arrythmia Features
The mean 24‐h PVC burden was 16.26% ± 8.68% of the total beat count. Ten (10.64%) patients had multifocal PVCs. The most frequent morphology of PVCs (Table 3) was LBBB with inferior axis 54 (57.45%). Among patients with RBBB morphology, 18 (19.15%) had the inferior axis and 16 (17.02%) patients had the superior axis. Two patients had previous ablation (one for typical atrial flutter and another for atrioventricular nodal reentrant tachycardia).
TABLE 3.
Arrhythmia features.
| PVC burden, % of total beat count on 24 h | 16.26% ± 8.68% |
| Multifocal PVCs | 10 (10.64%) |
| Previous ablation | 2 (2.13%) |
| 12‐lead ECG morphology of the dominant PVC | |
| LBBB inferior axis | 54 (57.45%) |
| LBBB superior axis | 6 (6.38%) |
| RBBB inferior axis | 18 (19.15%) |
| RBBB superior axis | 16 (17.02%) |
| Other characteristics of PVCs | |
| QRS width, ms | 146 ± 17 |
| Coupling interval, ms | 477 ± 91 |
Note: Values are mean ± SD or n (%).
Abbreviations: LBBB, left bundle branch block; PVCs, premature ventricular contractions; RBBB, right bundle branch block.
3.3. CMR Findings
All patients had biventricular volumes and function within normal 25th to 75th percentile (Table 4). A total of 67 (71.23%) patients had evidence of areas of LGE either in the left ventricle (n = 55), the right ventricle (n = 4), or both (n = 8). The median amount of LGE (percentage of ventricular mass) was 5% (25th to 75th percentile: 3.25% to 10.75%). The most common localization of scar was in the interventricular septum in 19 patients (28.35%), the inferolateral wall in 14 patients (20.9%), and the anterolateral wall in 11 patients (14.42%). Out of the 67 patients presenting with areas of LGE, two patients had an ischemic pattern suggestive of old embolic myocardial infarction.
TABLE 4.
CMR findings.
| Left ventricle | |
| EDV index, mL/m2 | 73 ± 14 |
| ESV index, mL/m2 | 23 ± 7 |
| EF, % | 61 ± 8 |
| Mass index, g/m2 | 60 ± 10 |
| LGE | 63 (67%) |
| Scar volume, % LV mass | 5 (3.25–10.75) |
| The most common localization of scar | |
| Basal interventricular septum | 19 (28.35%) |
| Anterior | 7 (10.4%) |
| Middle | 17 (25.3%) |
| Posterior | 11 (16.41%) |
| Inferolateral wall | 14 (20.9%) |
| Anterolateral wall | 11 (14.42%) |
| Right ventricle | |
| EDV index, mL/m2 | 85 ± 13 |
| ESV index, mL/m2 | 30 ± 10 |
| EF, % | 55 ± 10 |
| LGE | 4 (5.9%) |
Note: Values are mean ± SD, n (%), and n (25th to 75th percentile).
Abbreviations: CMR, cardiac magnetic resonance; EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; LGE, late gadolinium enhancement; LV, left ventricle.
3.4. Correlation of PVC Morphology, Clinical Characteristic, and Imaging Findings
The prevalence of areas of LGE identified by CMR was higher among patients with LBBB inferior axis morphology compared to others PVCs morphologies (n = 43 (64.18%) vs. n = 24 (35.82%); p < 0.05). Forty‐seven 47 (78.33%) patients with PVCs with LBBB morphology had evidence of LGE on CMR compared to 20 (58.82%) patients with RBBB morphology (p < 0.05) (Figure 2). There was no significant statistical difference between the patients with LBBB inferior axis compared to patients with LBBB superior axis (p = 0.46); eight out of the 10 patients with multifocal PVCs had scarring on CMR. There was no difference between the presence of LGE in patients with QRS fragmentation on ECG versus those without QRS fragmentation in sinusal beats (p = 0.4).
FIGURE 2.
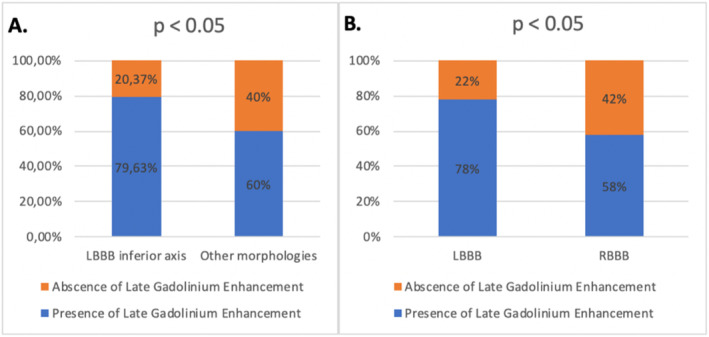
Prevalence of late gadolinium enhancement according to arrhythmia morphology: (A) LBBB inferior axis and other morphologies, (B) bundle branch block pattern. CMR, cardiac magnetic resonance; LBBB, left bundle branch block; RBBB, right bundle branch block.
In univariate analysis, the predictors of LGE were: male sex (OR 5.506, 95% CI: 2.092–14.49, p < 0.05), age ≥ 50 years (OR 1.047, 95% CI: 1.015–1.08, p < 0.05), PVC burden (OR 1.922, 95% CI: 1.723–1.976, p < 0.05), and a LBBB inferior axis (OR 4.09, 95% CI: 1.584–10.565, p < 0.05).
In multivariate analysis, age (OR 1.059; 95% CI: 1.019–1.099; p < 0.05) and a PVC LBBB inferior axis pattern (OR: 4.605; 95% CI: 1.472–14.404; p < 0.05) were independently correlated with the presence of LGE found on CMR (Table 5).
TABLE 5.
Univariate and multivariate logistic regression analysis of baseline covariates correlated with LGE on CMR.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age > 50 years | 1.047 (1.015–1.08) | < 0.05 | 1.059 (1.019–1.099) | < 0.05 |
| Male sex | 5.506 (2.092–14.49) | < 0.05 | 3.296 (0.89–12.205) | 0.074 |
| PVC burden | 1.922 (1.723–1.976) | < 0.05 | 0.929 (0.861–1.001) | 0.054 |
| LBBB inferior axis | 4.09 (1.584–10.565) | < 0.05 | 4.605 (1.472–14.404) | < 0.05 |
Abbreviations: CI, confidence interval; LAVI, left atrial volume index; LBBB, left bundle branch block; OR, odds ratio; PVCs, premature ventricular contractions. Statistically significant correlations are presented in bold values.
3.5. Correlation Between the Presence of LGE and the Need for Ablation in Patients With PVCs
Patients were followed for a period of 12 months. As part of our study protocol, patients were examined every 3 months by a clinical examination, TTE, and 24‐h Holter monitoring. Patients were referred for ablation if they were symptomatic in the context of frequent PVCs (despite of antiarrhythmic use), were suspected of having tachycardiomyopathy, or had a PVC burden of > 20%/24 h. During follow‐up of 12 month, 34 (36.2%) patients underwent radiofrequency ablation. Acute procedural success was achieved in 33 cases (97%). Sustained VT was induced in none of cases. There was no periprocedural complication.
Among the patients with LGE on CMR, 22 (32.8%) required radiofrequency ablation versus 12 patients (44.4%) without scarring on CMR who required an ablation procedure, (p = 0.29) (Figure 3).
FIGURE 3.
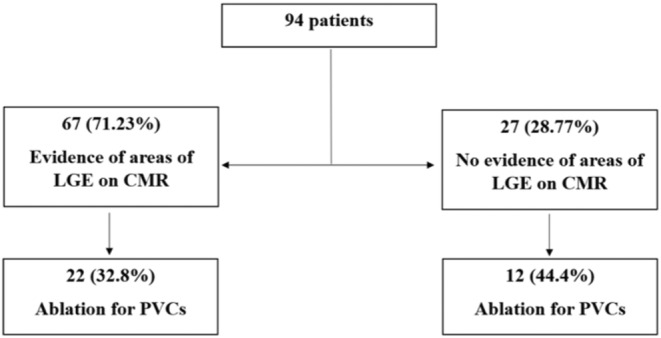
Long‐term outcome. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; PVCs, premature ventricular contractions.
The characteristics of the patients, the arrhythmia features, the presence of the scar on the CMR, and the need for ablation are depicted in Table 6.
TABLE 6.
Correlation between patient characteristics, arrhythmia features, presence of scar on CMR, and need for ablation.
| LGE on CMR | No LGE on CMR | p | |
|---|---|---|---|
| Age > 50 years | 51 (87.9%) | 7 (12.1%) | < 0.05 |
| Male sex | 53 (79.1%) | 14 (20.9%) | < 0.05 |
| PVC with LBBB morphology | 47 (78.33%) | 20 (21.67%) | < 0.05 |
| PVC with inferior axis | 52 (72.22%) | 20 (27.68%) | 0.71 |
| Symptomatic | 56 (73.68%) | 20 (26.32%) | 0.55 |
| Ablation | Yes (22 [32.8%]), No (45 [67.2%]) | Yes (12 [44.4%]), No (15 [55.6%]) | 0.29 |
Abbreviations: CMR, cardiac magnetic resonance; LBBB, left bundle branch block; LGE, late gadolinium enhancement; PVCs, premature ventricular contractions.
4. Discussion
Our study documents the prevalence of LGE identified by CMR imaging in patients without structural heart disease after the routine diagnostic workup (including ECG, echocardiography, and assessment of coronary arteries), in patients with frequent PVCs. Figure 4 demonstrates the presence of myocardial fibrosis by CMR in a patient with frequent PVCs who was included in the study.
FIGURE 4.
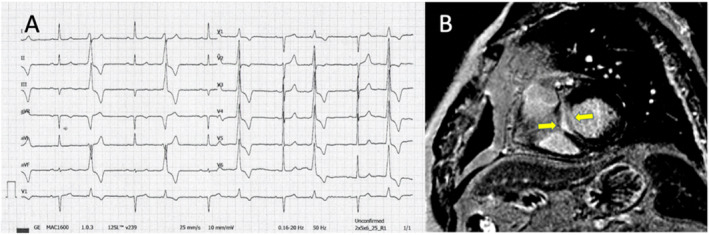
(A) ECG of a patients with 14,500 PVCs with LBBB morphology and inferior axis; (B) CMR imaging showing mid myocardial LGE at the level of the basal septum (nonischemic pattern).
The main findings are: (i) 71.23% of the patients with PVCs and apparently structurally normal heart present myocardial scarring (LGE) identified by CMR; (ii) the presence of LBBB with an inferior axis pattern of PVCs and male sex and age ≥ 50 years are correlated with the presence of myocardial scarring; (iii) patients with myocardial scar identified by CMR tend to be more symptomatic; (iv) Age and a PVC morphology of LBBB inferior axis are independently correlated with the presence of LGE on CMR; (v) The presence of LGE on CMR is not correlated with the need for ablation for PVCs. This highlights the limitations of the standard diagnostic tests and highlights the clinical utility of CMR in identifying potential hidden cardiomyopathic substrates.
4.1. CMR Findings in Individuals With Apparently Idiopathic PVCs
In the last decades, CMR has proven to be a valuable imaging tool for various cardiac conditions, demonstrating the ability to precisely and reliably evaluate ventricular volumes, structure, and function (Ganesan et al. 2018). There is limited information on the clinical usefulness of CMR imaging in individuals who exhibit frequent PVCs without underlying structural heart disease identified by routine investigations. These patients have traditionally been considered at low‐risk for cardiovascular events or sudden cardiac death (SCD) based on past longitudinal studies that showed minimal or no divergence in outcomes from the general population (Kennedy et al. 1985). Our group previously discussed this in a narrative review (Ailoaei et al. 2024).
There are several studies investigating the use of CMR in patients with idiopathic PVCs. The study conducted by Muser et al. (2020) included 518 patients identified predominantly through athlete screening (which may limit the generalizability of study) with frequent PVCs and negative routine diagnostic workup. In this study, myocardial abnormalities were detected in 85 (16%) of the patients, the presence of these abnormalities was significantly associated with male gender (odds ratio [OR]: 4.28; 95% confidence interval [CI]: 2.06–8.93; p = 0.01), a family history of sudden cardiac death and/or cardiomyopathy (OR: 3.61; 95% CI: 1.33–9.82; p = 0.01), multifocal PVCs (OR: 11.12; 95% CI: 4.35–28.46; p < 0.01), and non‐LBBB inferior axis morphology (OR: 14.11; 95% CI: 7.35–27.07; p < 0.01) (Muser et al. 2020). Another study conducted by Hosseini et al. (2022) included 255 patients with a wide range of PVC burden and LVEF. 35 patients (13.7%) had evidence of myocardial abnormality on CMR, and the independent predictors were age ≥ 60 years (OR: 6.96; 95% CI: 1.30–37.18), multifocal PVCs (OR: 10.90; 95% CI: 3.21–36.97), and non‐outflow tract left ventricular PVC origin (OR: 3.00; 95% CI: 1.00–8.95) (Hosseini et al. 2022).
In our study. 71.23% of the patients had evidence of LGE by CMR. This percentage is much higher than the one reported by the previously mentioned studies, probably secondary to a selection bias, since our cohort was selected from a tertiary center for arrhythmias that included mostly symptomatic patients. Additionally, in line with the literature, PVCs with LBBB inferior axis pattern, age, and male sex were associated with the presence of abnormalities found on CMR.
4.2. Prognostic Value of CMR Scarring in Patients With Idiopathic PVCs
Myocardial scarring detected by CMR in patients presenting with apparently idiopathic PVCs has an important practical value in terms of risk stratification as has been shown in previous studies (Muser et al. 2020; Hosseini et al. 2022; Nikolaidou et al. 2021).
In the study conducted by Muser et al. (2020), following a median follow‐up period of 67 months, a composite endpoint, which included SCD, resuscitated cardiac arrest, nonfatal episodes of ventricular fibrillation (VF), or sustained ventricular tachycardia (VT) necessitating appropriate implantable cardioverter‐defibrillator therapy occurred in 26 patients (5%, p < 0.01). The occurrence of this composite outcome was notably higher among individuals with myocardial abnormalities in CMR, with 25 individuals (29%) experiencing the outcome, in contrast to only 1 individual (0.2%) without such abnormalities (p < 0.01) (Muser et al. 2020).
This was confirmed also by Hosseini et al. (2022) after a median follow‐up of 36 months, the composite outcome (all‐cause mortality, nonfatal episode of VF or sustained VT requiring intervention, and reduction in LVEF of ≥ 10% compared to baseline LVEF) occurred in 15 (5.9%) patients. The presence of a myocardial abnormality on CMR was independently associated with the composite outcome (HR: 4.35; 95% CI: 1.34–14.15; p = 0.014) (Hosseini et al. 2022).
However, our study did not show a correlation between the presence of LGE on CMR and the need for ablation (p = 0.29). This is probably due to the inclusion bias in the study as well as the too small group of patients included in the study. In the future, we plan a long‐term follow‐up to study if the presence of the scar detected by CMR imaging correlates with mortality from cardiac causes, the development of cardiomyopathy, the need for ICD implantation, and malignant ventricular arrhythmias.
4.3. Clinical Implication
The risk of major adverse cardiovascular events in patients with frequent PVCs and no structural heart disease is considered to be low (Lee et al. 2019; Anderson et al. 2019). Nonetheless, a minority of patients may encounter unfavorable clinical consequences, underscoring the necessity to stratify patients based on the risk of such outcomes (Muser et al. 2020; Hosseini et al. 2022; Nikolaidou et al. 2021).
Risk models for adverse outcomes in this population are currently scarce (Voskoboinik et al. 2020; Thibert et al. 2021), and the identification of individuals at elevated risk for unfavorable clinical events may guide the implementation of more assertive treatments to avert such occurrences.
Our study supports the use of CMR in all patients with frequent PVCs, particularly in males > 50 years old with LBBB inferior axis pattern PVCs. Moreover, in our cohort, based on the CMR results, genetic testing was discussed with a total of 21 LGE positive patients. Out of these 21 patients, genetic testing was performed in 13 patients. Six patients had pathogenic mutations (two DSP, two TTN, one FLNC, and one PLN) that led to family screening and personalized treatment. Data regarding these findings will be disseminated by our group in future research papers.
4.4. Study Limitations
This was a single center study that included patients addressed to our tertiary center for the management of arrhythmias. As such, the characteristics of the patient population might have been affected by selection and/or referral bias. This lack of standardization may have introduced unmeasured confounding variables, potentially leading to an overestimation of the prevalence of abnormalities on CMR within our sample. Electrophysiological study was not routinely performed, therefore, a robust correlation between the origin of PVCs and the location of myocardial abnormalities identified by CMR could not be established.
5. Conclusions
Our study shows that the majority of the patients with frequent PVCs and apparently structurally normal heart present myocardial scarring identified by CMR. PVCs with LBBB inferior axis pattern, age ≥ 50 years, and male sex are associated with the presence of abnormalities on CMR. In view of the growing body of evidence suggesting that CMR may hold a prognostic role in patients with PVCs, with diagnostic and therapeutical implications, more studies are needed for definitive conclusions.
Author Contributions
Conceptualization: S.A., N.S. and C.U.; methodology: C.S., R.A.S., M.G.; validation: R.A.S., C.S. and L.S.; formal analysis: N.S.; investigation: G.S.; resources: C.U.; data curation: C.U.; writing – original draft preparation: N.S., S.A., C.U.; writing – review and editing: C.U., N.S., S.A., M.G., R.A.S.; visualization: A.C., R.A.S., L.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, C.U. During the preparation of this work, the authors did not use any AI or AI‐assisted technologies.
References
- Ailoaei, S. , Sorodoc L., Ureche C., et al. 2024. “Role of Cardiac Magnetic Resonance in the Assessment of Patients With Premature Ventricular Contractions: A Narrative Review.” Anatolian Journal of Cardiology 28: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, R. D. , Kumar S., Parameswaran R., et al. 2019. “Differentiating Right‐ and Left‐Sided Outflow Tract Ventricular Arrhythmias.” Circulation. Arrhythmia and Electrophysiology 12: e007392. [DOI] [PubMed] [Google Scholar]
- Ephrem, G. , Levine M., Friedmann P., and Schweitzer P.. 2013. “The Prognostic Significance of Frequency and Morphology of Premature Ventricular Complexes During Ambulatory Holter Monitoring.” Annals of Noninvasive Electrocardiology 18: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, A. N. , Gunton J., Nucifora G., McGavigan A. D., and Selvanayagam J. B.. 2018. “Impact of Late Gadolinium Enhancement on Mortality, Sudden Death and Major Adverse Cardiovascular Events in Ischemic and Nonischemic Cardiomyopathy: A Systematic Review and Meta‐Analysis.” International Journal of Cardiology 254: 230–237. [DOI] [PubMed] [Google Scholar]
- Haruta, D. , Akahoshi M., Hida A., et al. 2016. “Prognostic Significance of Premature Ventricular Contractions Without Obvious Heart Diseases Determined by Standard 12‐Lead Electrocardiography Considering Their Morphology.” Annals of Noninvasive Electrocardiology 21: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, F. , Thibert M. J., Gulsin G. S., et al. 2022. “Cardiac Magnetic Resonance in the Evaluation of Patients With Frequent Premature Ventricular Complexes.” JACC: Clinical Electrophysiology 8: 1122–1132. [DOI] [PubMed] [Google Scholar]
- Kennedy, H. L. , Whitlock J. A., Sprague M. K., Kennedy L. J., Buckingham T. A., and Goldberg R. J.. 1985. “Long‐Term Follow‐Up of Asymptomatic Healthy Subjects With Frequent and Complex Ventricular Ectopy.” New England Journal of Medicine 312: 193–197. [DOI] [PubMed] [Google Scholar]
- Kramer, C. M. , Barkhausen J., Bucciarelli‐Ducci C., Flamm S. D., Kim R. J., and Nagel E.. 2020. “Standardized Cardiovascular Magnetic Resonance Imaging (CMR) Protocols: 2020 Update.” Journal of Cardiovascular Magnetic Resonance 22: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, R. M. , Badano L. P., Mor‐Avi V., et al. 2015. “Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update From the American Society of Echocardiography and the European Association of Cardiovascular Imaging.” Journal of the American Society of Echocardiography 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- Lee, A. K. Y. , Andrade J., Hawkins N. M., et al. 2019. “Outcomes of Untreated Frequent Premature Ventricular Complexes With Normal Left Ventricular Function.” Heart 105: 1408–1413. [DOI] [PubMed] [Google Scholar]
- Marcus, G. M. 2020. “Evaluation and Management of Premature Ventricular Complexes.” Circulation 141: 1404–1418. [DOI] [PubMed] [Google Scholar]
- Maury, P. , Rollin A., Mondoly P., and Duparc A.. 2015. “Management of Outflow Tract Ventricular Arrhythmias.” Current Opinion in Cardiology 30: 50–57. [DOI] [PubMed] [Google Scholar]
- Muser, D. , Santangeli P., Castro S. A., et al. 2020. “Risk Stratification of Patients With Apparently Idiopathic Premature Ventricular Contractions.” JACC: Clinical Electrophysiology 6: 722–735. [DOI] [PubMed] [Google Scholar]
- Muser, D. , Santangeli P., Selvanayagam J. B., and Nucifora G.. 2018. “Role of Cardiac Magnetic Resonance Imaging in Patients With Idiopathic Ventricular Arrhythmias.” Current Cardiology Reviews 15: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muser, D. , Tritto M., Mariani M. V., et al. 2021. “Diagnosis and Treatment of Idiopathic Premature Ventricular Contractions: A Stepwise Approach Based on the Site of Origin.” Diagnostics (Basel) 11: 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, K. T. , Vittinghoff E., Dewland T. A., et al. 2017. “Ectopy on a Single 12‐Lead ECG, Incident Cardiac Myopathy, and Death in the Community.” Journal of the American Heart Association 6: e006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidou, C. , Kotanidis C. P., Wijesurendra R., et al. 2021. “Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients With Frequent Idiopathic Ventricular Arrhythmias.” Diagnostics (Basel) 11: 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone, B. , Muser D., Casella M., et al. 2019. “Detection of Concealed Structural Heart Disease by Imaging in Patients With Apparently Idiopathic Premature Ventricular Complexes: A Review of Current Literature.” Clinical Cardiology 42: 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichand, S. , Killu A. M., Padmanabhan D., et al. 2017. “Incidence of Idiopathic Ventricular Arrhythmias.” Circulation. Arrhythmia and Electrophysiology 10: e004662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgente, A. , Farkowski M. M., Iliodromitis K., et al. 2022. “Contemporary Clinical Management of Monomorphic Idiopathic Premature Ventricular Contractions: Results of the European Heart Rhythm Association Survey.” EP Europace 24: 1006–1014. [DOI] [PubMed] [Google Scholar]
- Thibert, M. J. , Hosseini F., Andrade J. G., Hawkins N. M., and Deyell M. W.. 2021. “External Validation of the ABC‐VT Risk Score for Patients With Frequent Ventricular Ectopy.” Circulation. Arrhythmia and Electrophysiology 14: e009952. [DOI] [PubMed] [Google Scholar]
- Voskoboinik, A. , Hadjis A., Alhede C., et al. 2020. “Predictors of Adverse Outcome in Patients With Frequent Premature Ventricular Complexes: The ABC‐VT Risk Score.” Heart Rhythm 17: 1066–1074. [DOI] [PubMed] [Google Scholar]
- You, C. X. , and Liu C. F.. 2019. “Premature Ventricular Contractions and Cardiomyopathy.” Cardiology in Review 27: 322–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, C.U. During the preparation of this work, the authors did not use any AI or AI‐assisted technologies.


