Abstract
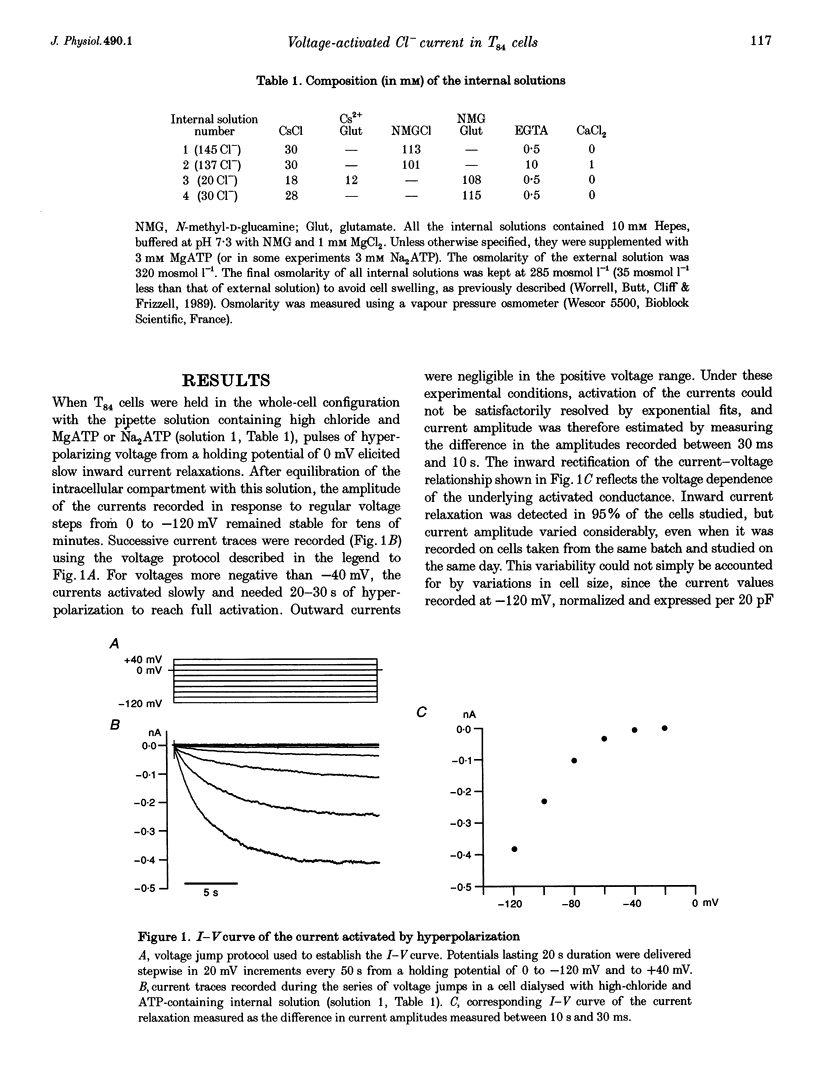
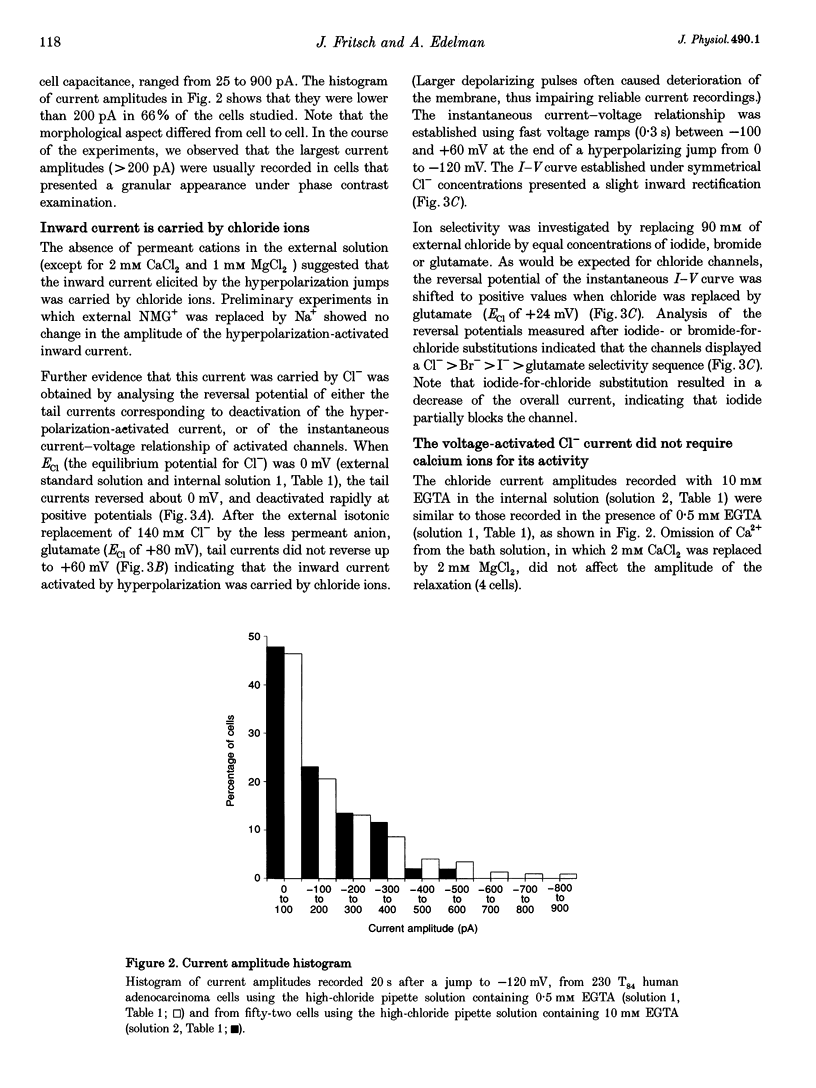
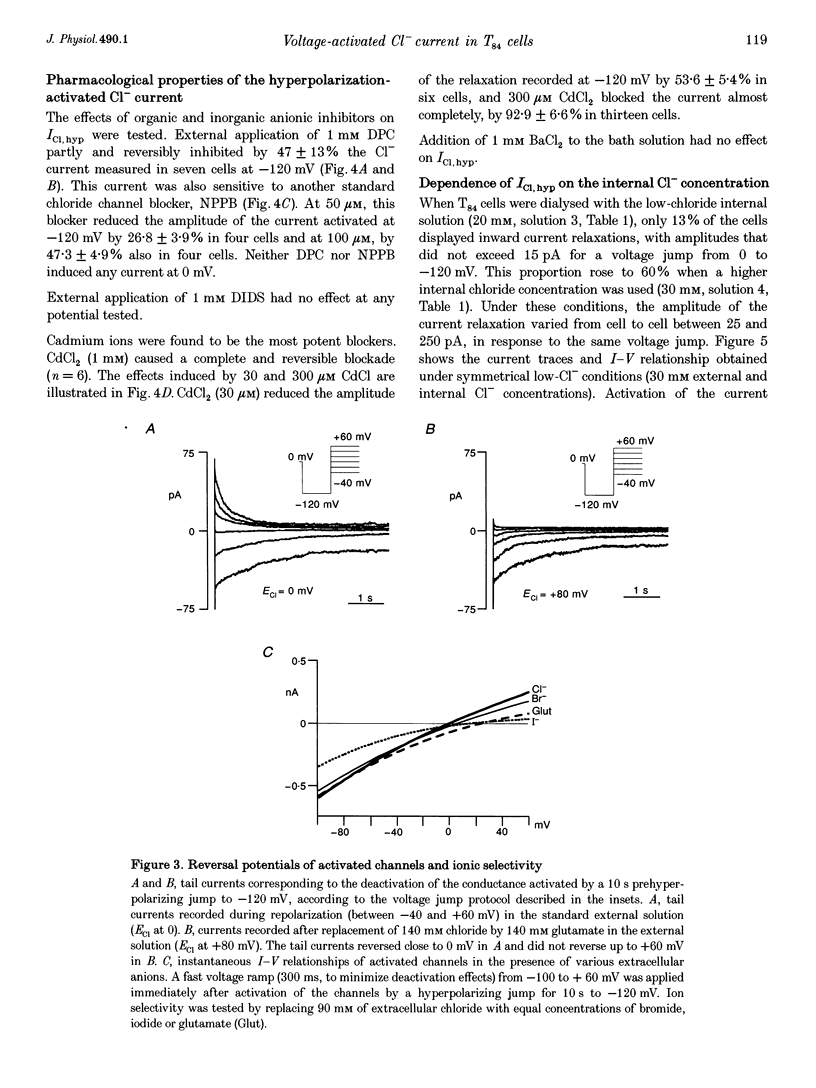
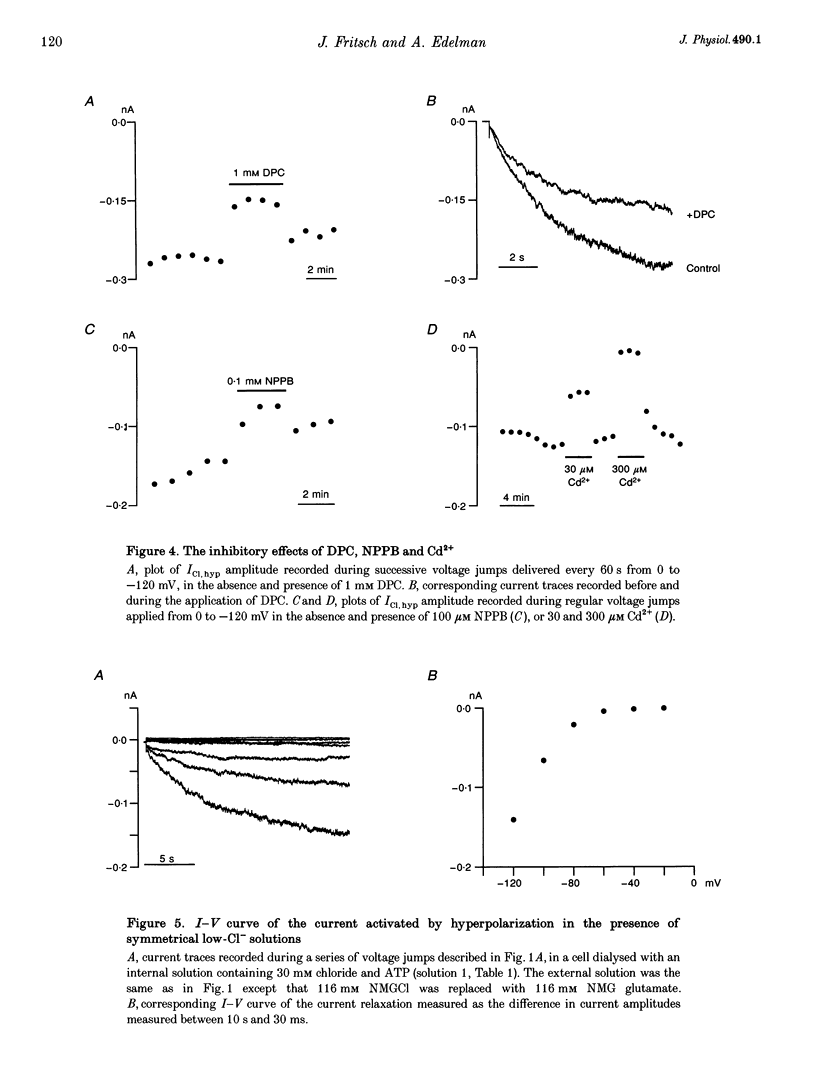
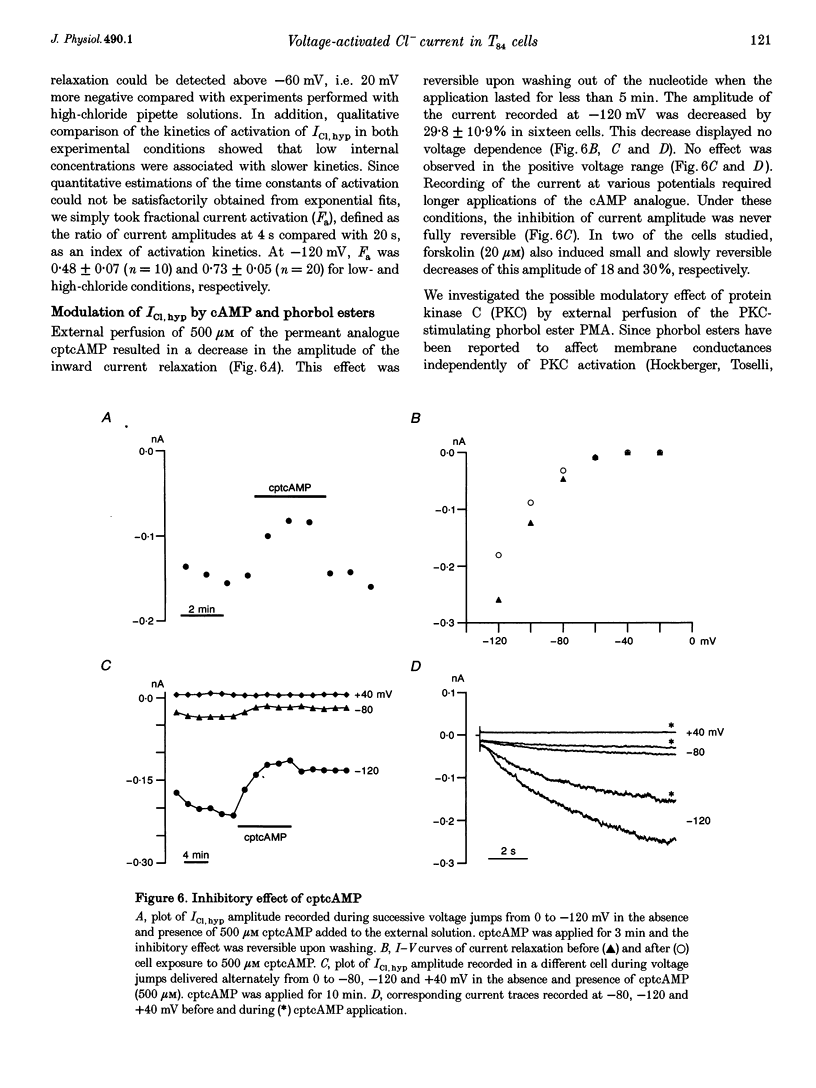
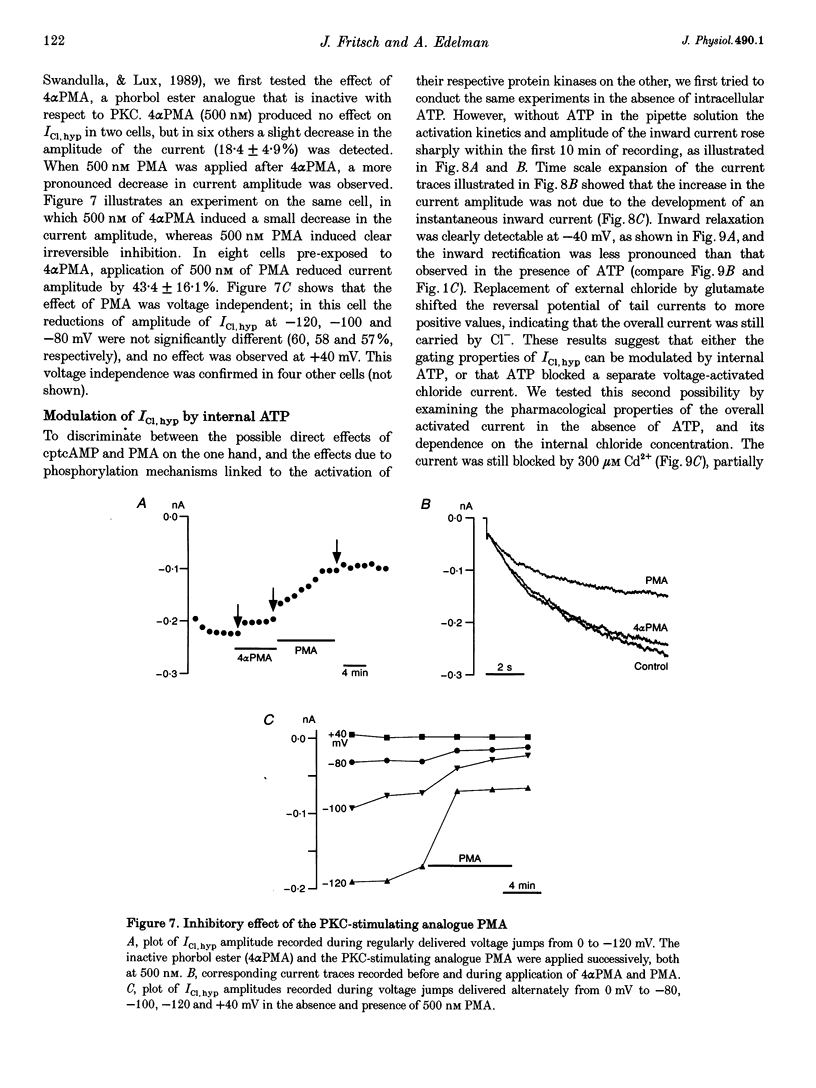
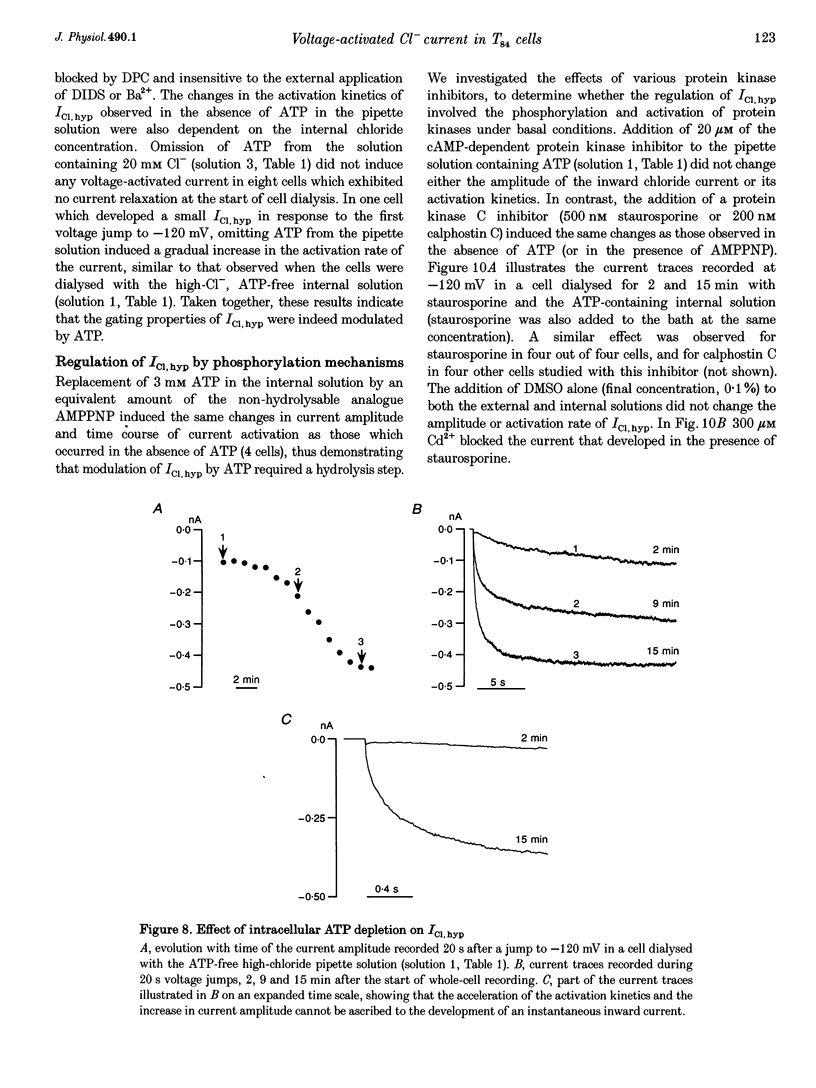
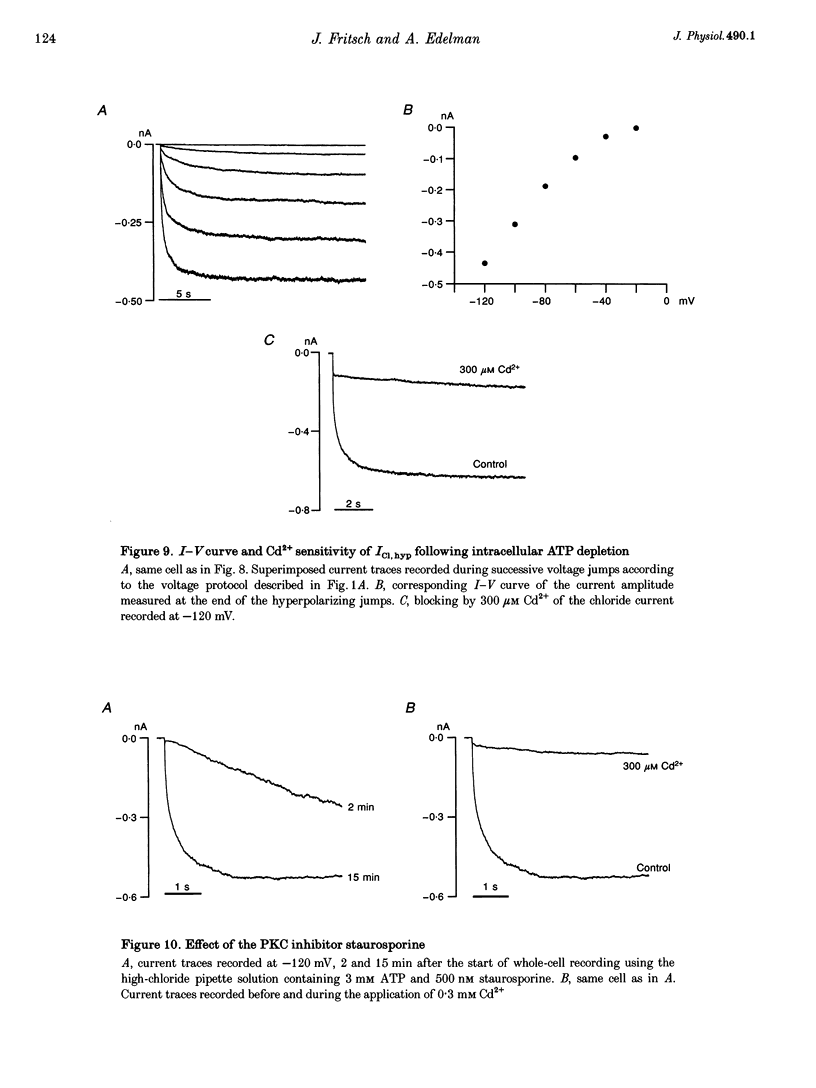
1. Hyperpolarization-activated Cl- currents (ICl,hyp) were investigated in the T84 human adenocarcinoma cell line, using the patch-clamp whole-cell configuration. 2. During whole-cell recording with high-chloride and ATP-containing internal solutions, hyperpolarizing jumps from a holding potential of 0 mV elicited slow inward current relaxations, carried by Cl- and detected at membrane potentials more negative than -40 mV. Analysis of the relative permeabilities to monovalent anions gave the following sequence: Cl- > Br- > I- > glutamate. 3. ICl,hyp was partially inhibited by 1 mM diphenylamine-2-carboxylic acid or 0.1 mM 5-nitro-2-(3-phenylpropylamino)-benzoate, and was completely blocked by Cd2+ (> 300 microM). It was insensitive to 1 mM external 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid or 1 mM Ba2+. 4. ICl,hyp was inhibited by external application of 500 microM cptcAMP (8-(4-chlorophenylthio)-adenosine 3':5'-cyclic monophosphate) or 500 nM of the protein kinase C activator, phorbol 12-myristate, 13-acetate. 5. (i) Omission of ATP from the pipette solution, (ii) ATP replacement by the non-hydrolysable ATP analogue 5'-adenylylimidodiphosphate, and (iii) inhibition of protein kinase C by staurosporine or calphostin C accelerated the activation kinetics of the current and increased its amplitude, but did not alter its pharmacological properties. 6. We conclude that hyperpolarization-activated Cl- channels similar to those of ClC-2 channels (mammalian homologue of Torpedo chloride channel ClC-0) are present in T84 cells, and that their gating properties are modulated by phosphorylation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton E. W., Manning S. D., Schlatter P. J., Geddes D. M., Williams A. J. Characterization of a Ca(2+)-dependent anion channel from sheep tracheal epithelium incorporated into planar bilayers. J Physiol. 1991 Nov;443:137–159. doi: 10.1113/jphysiol.1991.sp018827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B., Guillemare E., Lesage F., Honoré E., Romey G., Lazdunski M., Barhanin J. The protein IsK is a dual activator of K+ and Cl- channels. Nature. 1993 Oct 28;365(6449):850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Barrett K. E. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C859–C868. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- Block M. L., Moody W. J. A voltage-dependent chloride current linked to the cell cycle in ascidian embryos. Science. 1990 Mar 2;247(4946):1090–1092. doi: 10.1126/science.2309122. [DOI] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Characterization of a chloride conductance activated by hyperpolarization in Aplysia neurones. J Physiol. 1983 Sep;342:277–308. doi: 10.1113/jphysiol.1983.sp014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Fritsch J. Activation of hyperpolarization and atypical osmosensitivity of a Cl- current in rat osteoblastic cells. J Membr Biol. 1994 Jun;140(3):173–188. doi: 10.1007/BF00233706. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinudom A., Young J. A., Cook D. I. Na+ and Cl- conductances are controlled by cytosolic Cl- concentration in the intralobular duct cells of mouse mandibular glands. J Membr Biol. 1993 Sep;135(3):289–295. doi: 10.1007/BF00211100. [DOI] [PubMed] [Google Scholar]
- Field M., Semrad C. E. Toxigenic diarrheas, congenital diarrheas, and cystic fibrosis: disorders of intestinal ion transport. Annu Rev Physiol. 1993;55:631–655. doi: 10.1146/annurev.ph.55.030193.003215. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Benos D. J. CFTR! Am J Physiol. 1992 Aug;263(2 Pt 1):C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- Groschner K., Kukovetz W. R. Voltage-sensitive chloride channels of large conductance in the membrane of pig aortic endothelial cells. Pflugers Arch. 1992 Jun;421(2-3):209–217. doi: 10.1007/BF00374829. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Huflejt M. E., Blum R. A., Miller S. G., Moore H. P., Machen T. E. Regulated Cl transport, K and Cl permeability, and exocytosis in T84 cells. J Clin Invest. 1994 May;93(5):1900–1910. doi: 10.1172/JCI117181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J., Steinmeyer K., Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990 Dec 6;348(6301):510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Saigusa A., Tamura T. Blockade of Cl channels by organic and inorganic blockers in vascular smooth muscle cells. Pflugers Arch. 1991 Apr;418(3):204–213. doi: 10.1007/BF00370515. [DOI] [PubMed] [Google Scholar]
- Kowdley G. C., Ackerman S. J., John J. E., 3rd, Jones L. R., Moorman J. R. Hyperpolarization-activated chloride currents in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):217–230. doi: 10.1085/jgp.103.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman R. P., Chase H. S., Jr Protein kinase C does not participate in carbachol's secretory action in T84 cells. Am J Physiol. 1992 Jul;263(1 Pt 1):C140–C146. doi: 10.1152/ajpcell.1992.263.1.C140. [DOI] [PubMed] [Google Scholar]
- Lotshaw D. P., Levitan I. B. Serotonin and forskolin modulation of a chloride conductance in cultured identified Aplysia neurons. J Neurophysiol. 1987 Nov;58(5):922–939. doi: 10.1152/jn.1987.58.5.922. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986 Jun 12;321(6071):695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- McEwan G. T., Brown C. D., Hirst B. H., Simmons N. L. Characterisation of volume-activated ion transport across epithelial monolayers of human intestinal T84 cells. Pflugers Arch. 1993 May;423(3-4):213–220. doi: 10.1007/BF00374397. [DOI] [PubMed] [Google Scholar]
- McEwan G. T., Hirst B. H., Simmons N. L. Carbachol stimulates Cl- secretion via activation of two distinct apical Cl- pathways in cultured human T84 intestinal epithelial monolayers. Biochim Biophys Acta. 1994 Feb 17;1220(3):241–247. doi: 10.1016/0167-4889(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Palmer C. J., John J. E., 3rd, Durieux M. E., Jones L. R. Phospholemman expression induces a hyperpolarization-activated chloride current in Xenopus oocytes. J Biol Chem. 1992 Jul 25;267(21):14551–14554. [PubMed] [Google Scholar]
- Noulin J. F., Joffre M. Characterization and cyclic AMP-dependence of a hyperpolarization-activated chloride conductance in Leydig cells from mature rat testis. J Membr Biol. 1993 Apr;133(1):1–15. doi: 10.1007/BF00231873. [DOI] [PubMed] [Google Scholar]
- Overholt J. L., Saulino A., Drumm M. L., Harvey R. D. Rectification of whole cell cystic fibrosis transmembrane conductance regulator chloride current. Am J Physiol. 1995 Mar;268(3 Pt 1):C636–C646. doi: 10.1152/ajpcell.1995.268.3.C636. [DOI] [PubMed] [Google Scholar]
- Pahapill P. A., Schlichter L. C. Cl- channels in intact human T lymphocytes. J Membr Biol. 1992 Jan;125(2):171–183. doi: 10.1007/BF00233356. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. A calcium-independent chloride current activated by hyperpolarization in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):191–199. doi: 10.1098/rspb.1988.0018. [DOI] [PubMed] [Google Scholar]
- Pusch M., Jentsch T. J. Molecular physiology of voltage-gated chloride channels. Physiol Rev. 1994 Oct;74(4):813–827. doi: 10.1152/physrev.1994.74.4.813. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., La B. Q., Carosi S. L. Double-barreled chloride channels of collecting duct basolateral membrane. Am J Physiol. 1990 Jul;259(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1990.259.1.F46. [DOI] [PubMed] [Google Scholar]
- Saxon M. L., Zhao X., Black J. D. Activation of protein kinase C isozymes is associated with post-mitotic events in intestinal epithelial cells in situ. J Cell Biol. 1994 Aug;126(3):747–763. doi: 10.1083/jcb.126.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superdock K. R., Snyders D. K., Breyer M. D. ATP-inhibitable Cl- channel in apical membranes of cultured rabbit cortical collecting duct cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C957–C965. doi: 10.1152/ajpcell.1993.265.4.C957. [DOI] [PubMed] [Google Scholar]
- Taglietti V., Tanzi F., Romero R., Simoncini L. Maturation involves suppression of voltage-gated currents in the frog oocyte. J Cell Physiol. 1984 Dec;121(3):576–588. doi: 10.1002/jcp.1041210317. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Mintenig G. M., Sepúlveda F. V. Cl- currents of unstimulated T84 intestinal epithelial cells studied by intracellular recording. J Membr Biol. 1994 Feb;137(3):237–247. doi: 10.1007/BF00232592. [DOI] [PubMed] [Google Scholar]
- Walters R. J., Sepúlveda F. V. A basolateral K+ conductance modulated by carbachol dominates the membrane potential of small intestinal crypts. Pflugers Arch. 1991 Nov;419(5):537–539. doi: 10.1007/BF00370802. [DOI] [PubMed] [Google Scholar]


