Abstract
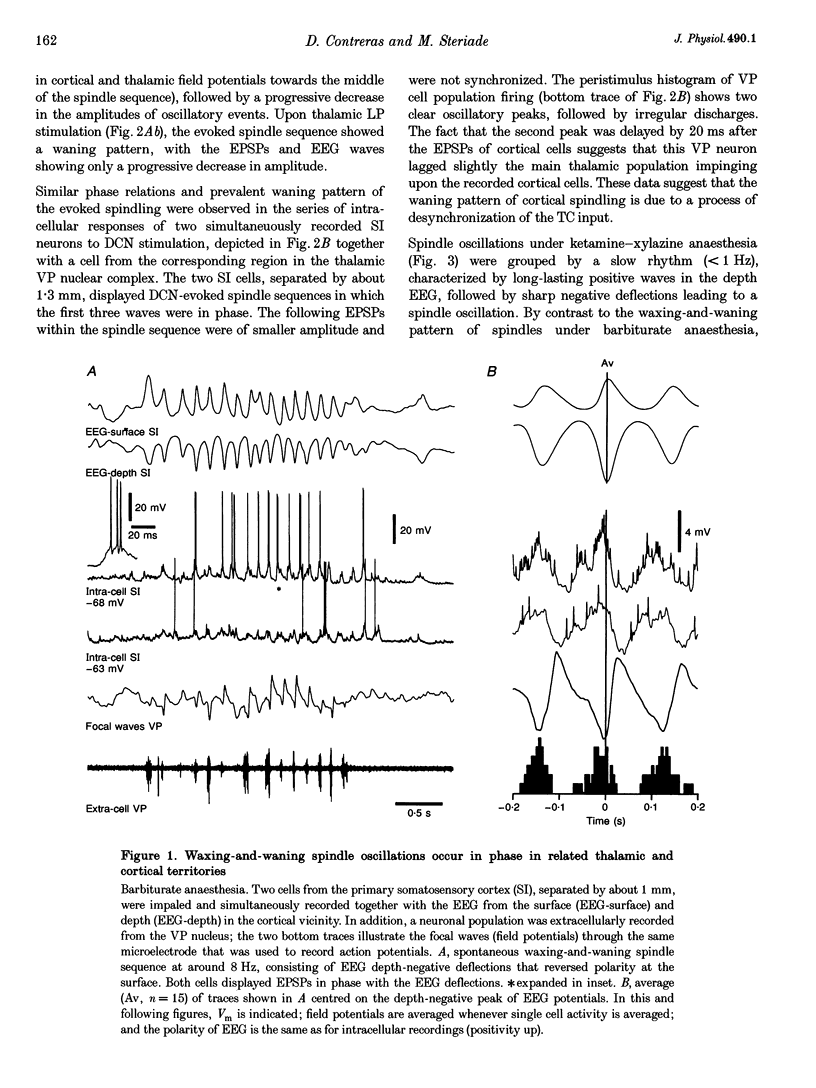
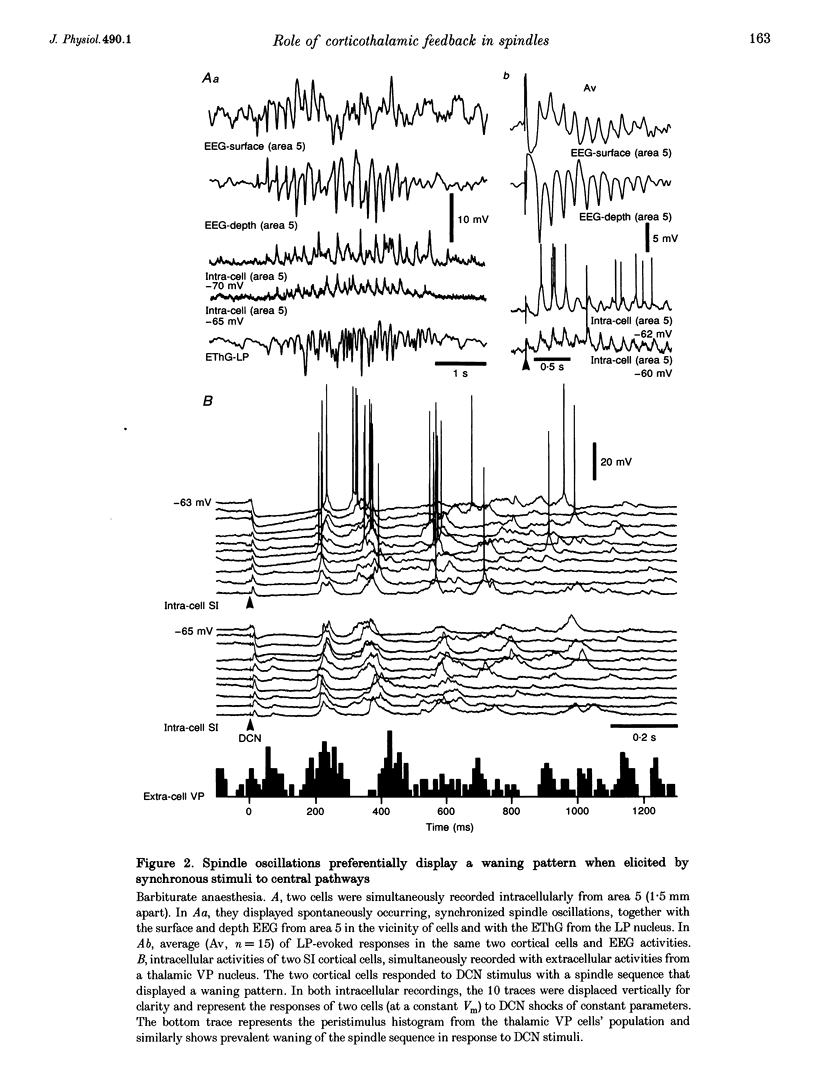
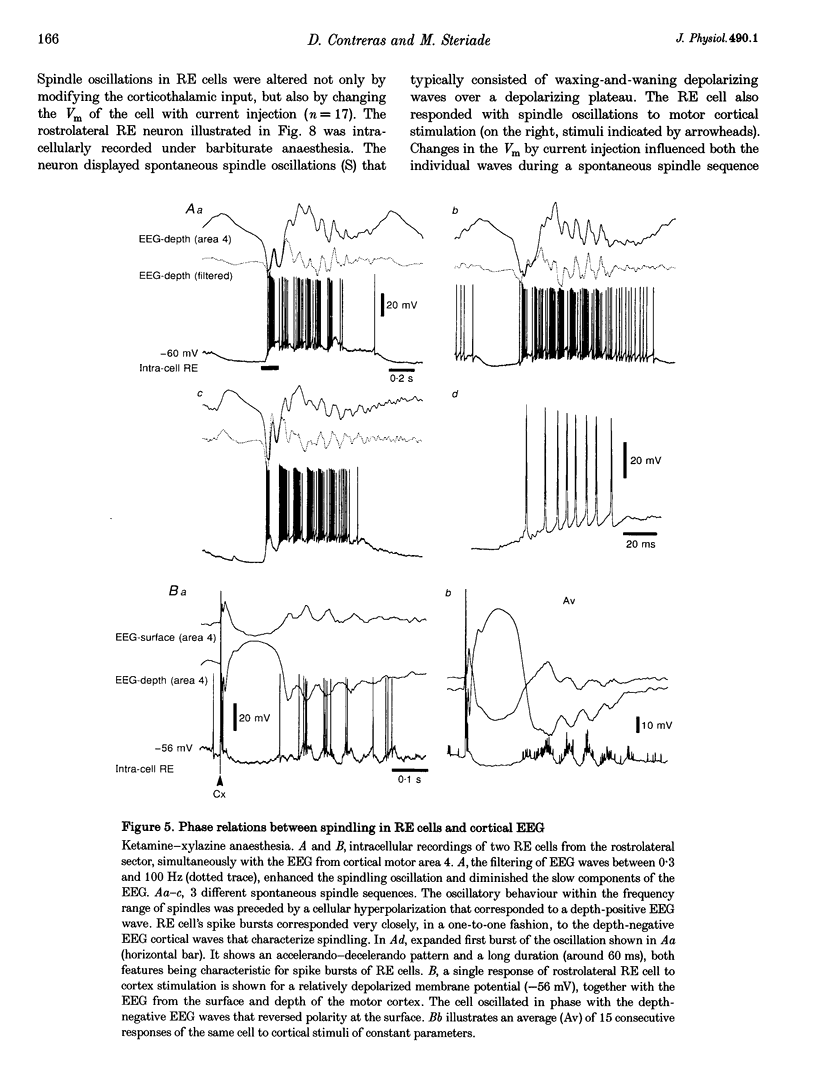
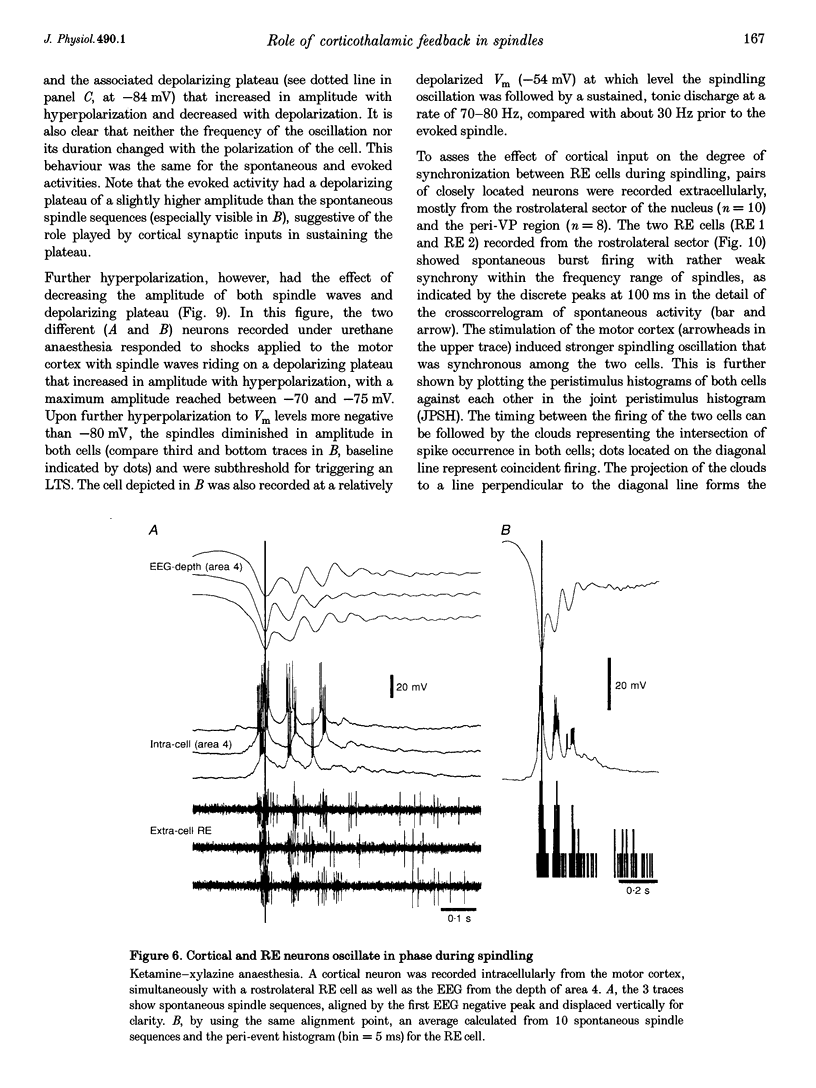
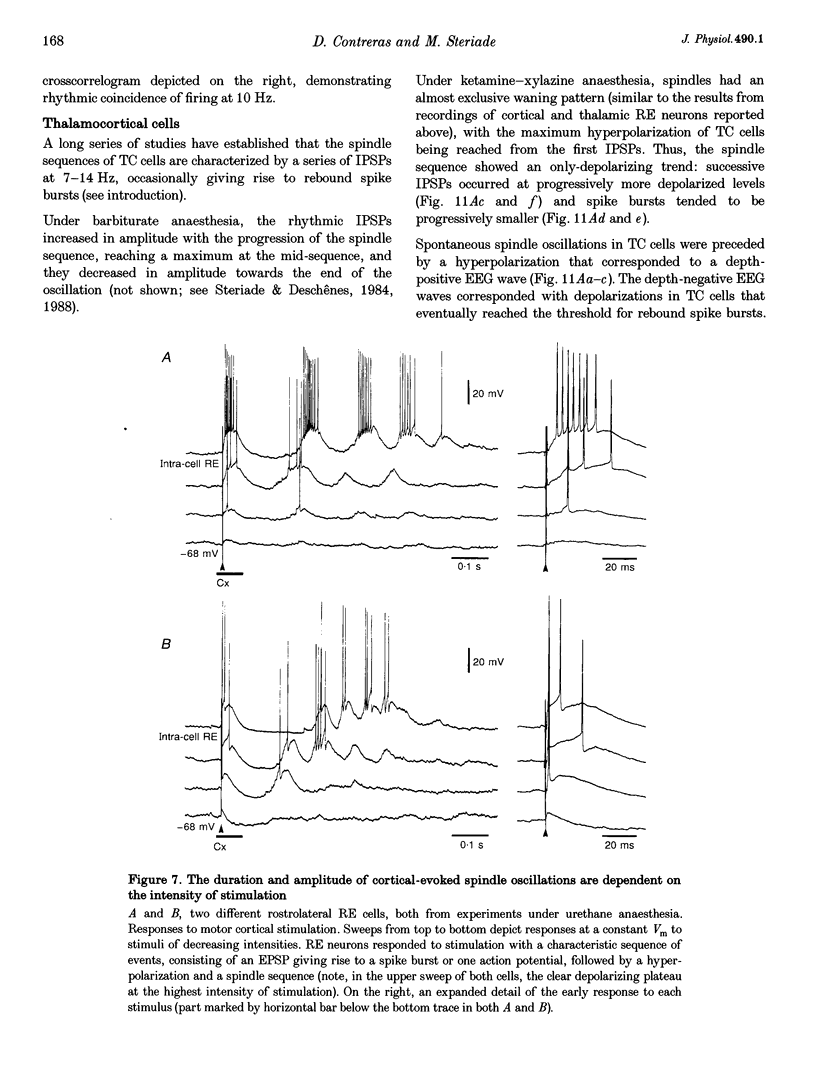
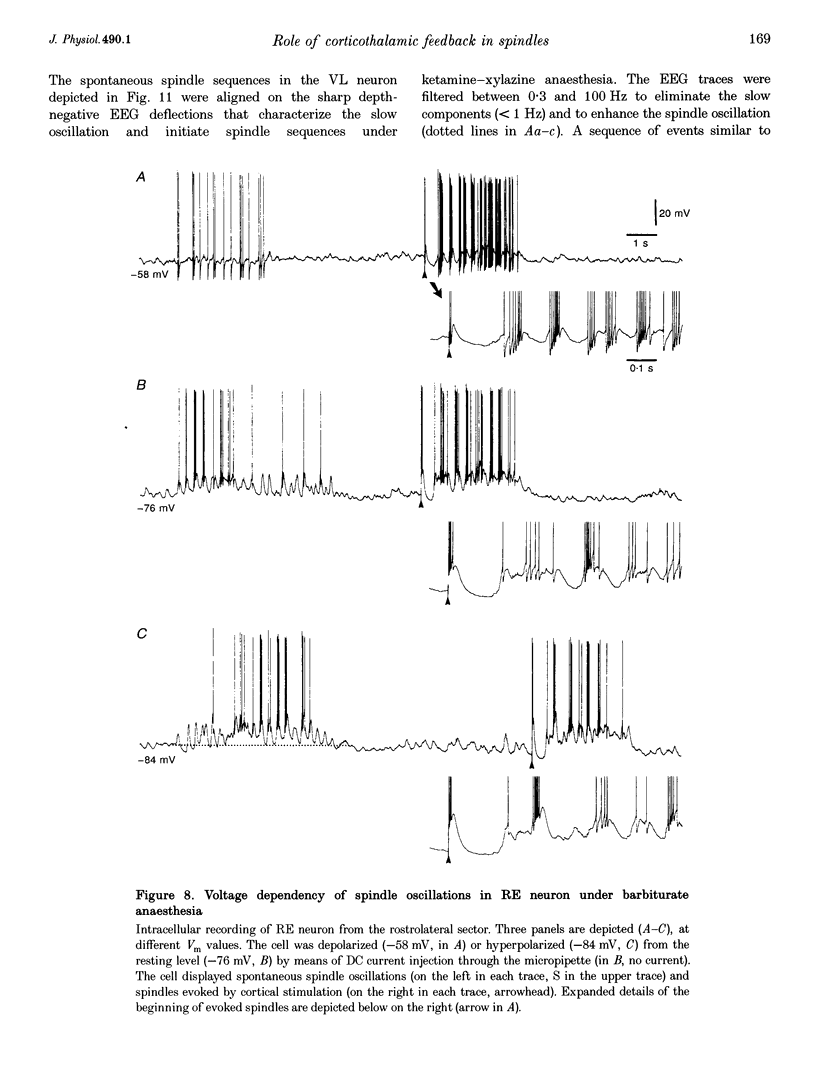
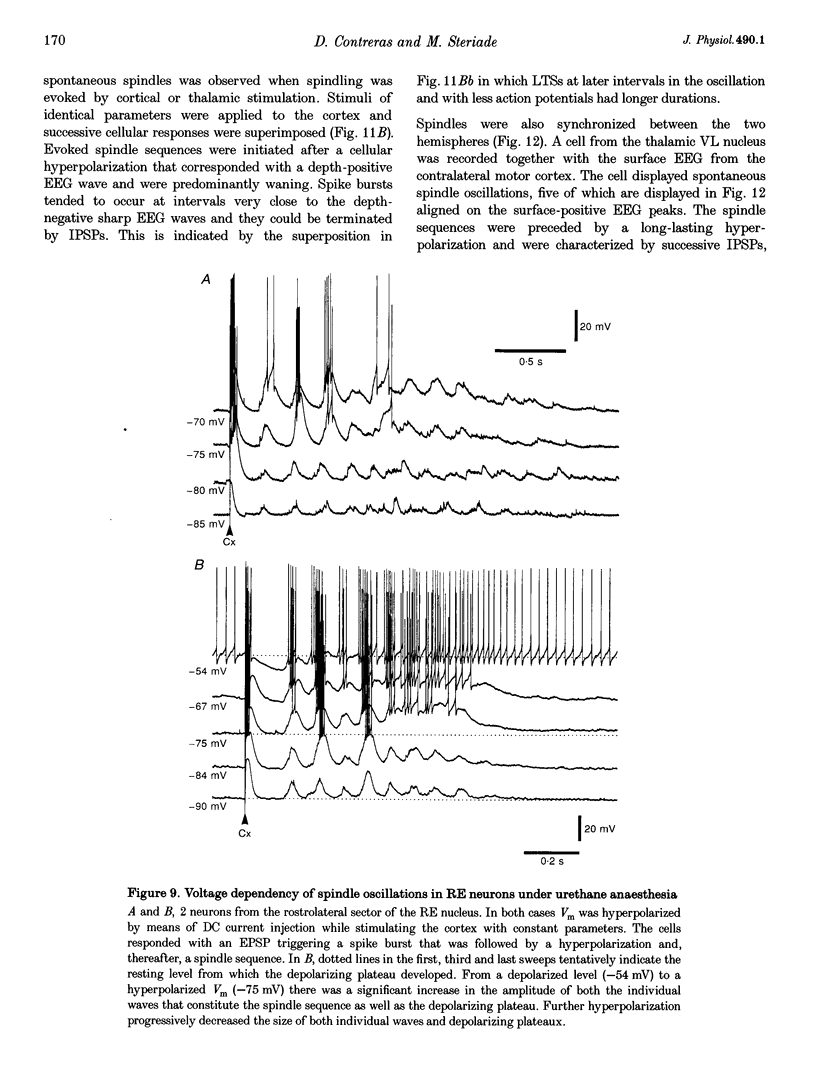
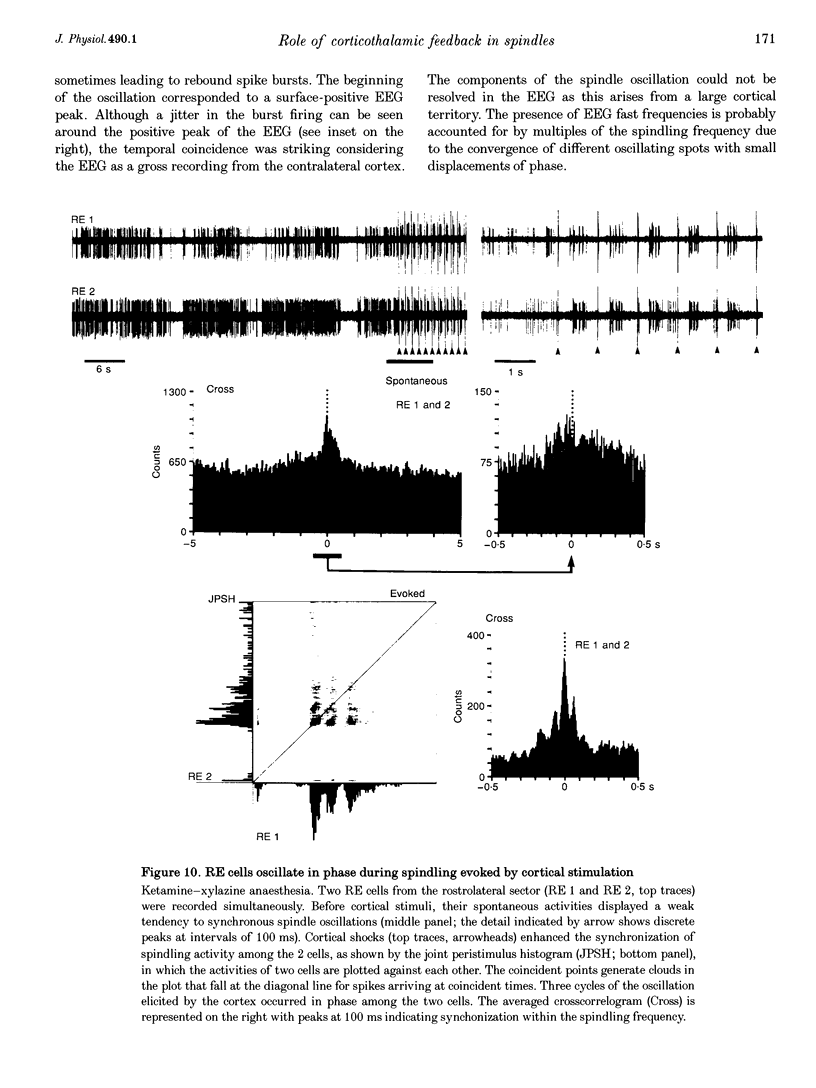
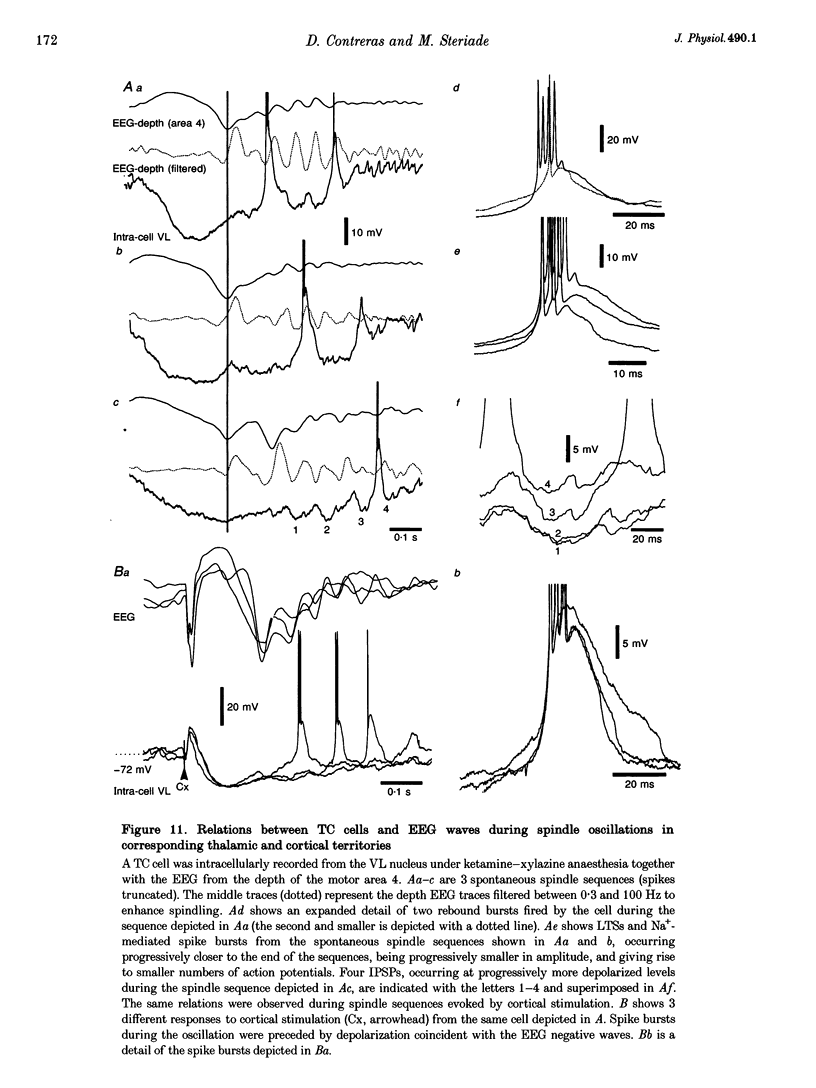
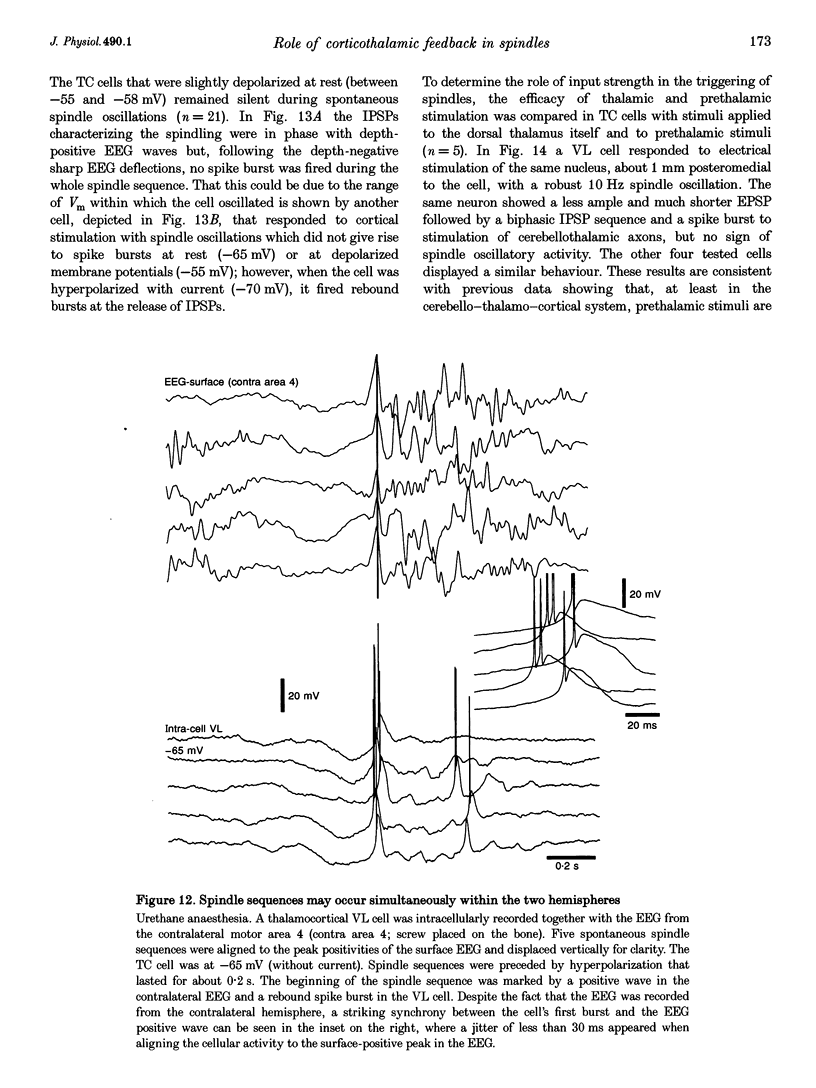
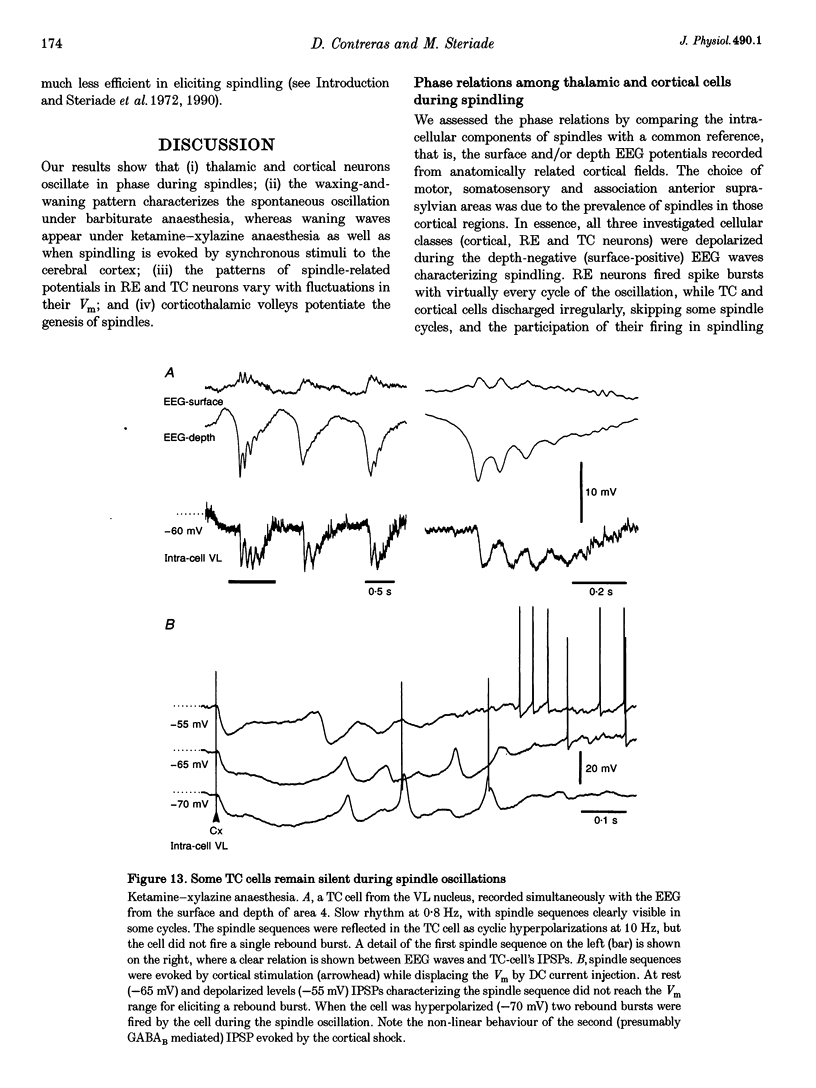
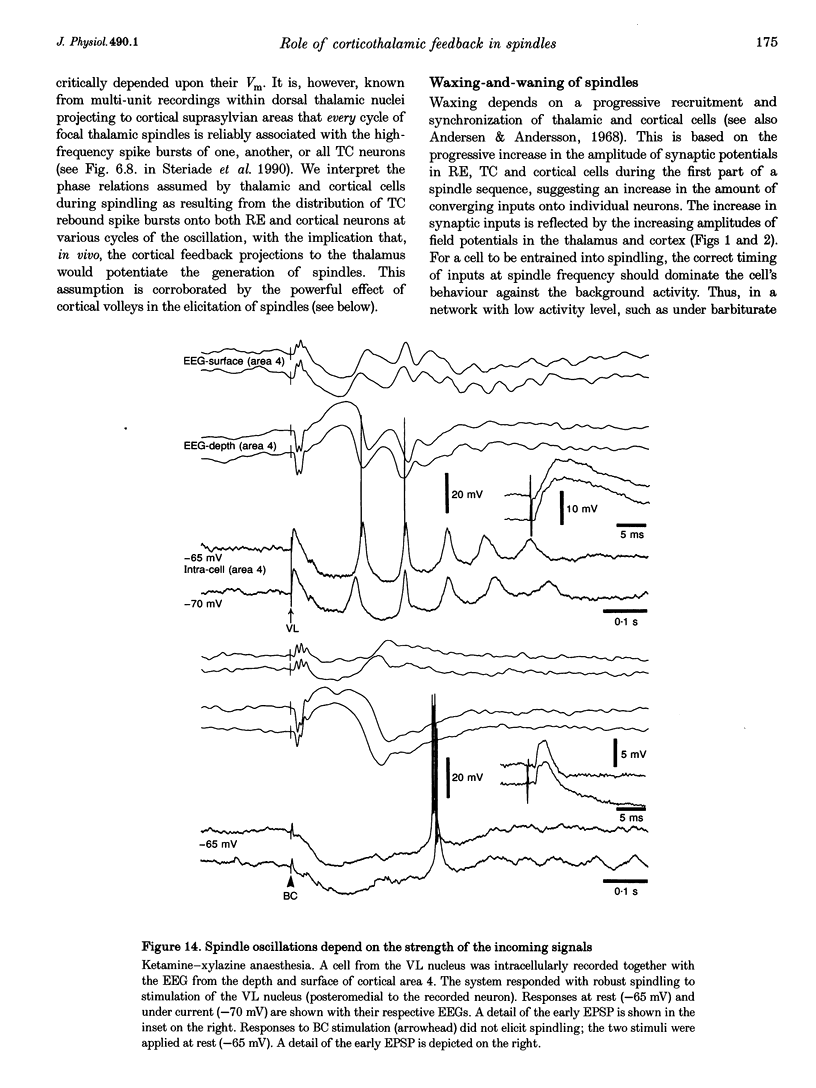
1. Spindles represent an oscillatory activity (7-14 Hz) of the electroencephalogram (EEG) originating in the thalamus and appearing during early stages of sleep. We investigated: (i) the phase relations between thalamic and cortical neurons during this rhythm; (ii) the patterns of spindles under different anaesthetics and their modifications at various levels of the membrane potential (Vm); and (iii) the potentiating role of the corticothalamic feedback in the genesis of spindles. Intra- and extracellular recordings were performed in cats from reticular and dorsal thalamic nuclei, as well as from various cortical areas. 2. In thalamic reticular neurons, spindles were sequences of waves at 7-14 Hz, riding on a prolonged depolarizing plateau and occurring in phase with depth-negative cortical EEG waves. In thalamocortical cells, spindles consisted of inhibitory postsynaptic potentials (IPSPs) in phase with depth-positive cortical EEG waves and occasionally leading to rebound spike bursts. In cortical cells, spindle waves were rhythmic (7-14 Hz) excitatory postsynaptic potentials (EPSPs) that sometimes gave rise to action potentials. Spindles occurred in phase among thalamic reticular, thalamocortical and neocortical neurons. 3. In thalamic reticular neurons, spindle waves and their depolarizing plateaux increased in amplitude with slight cellular hyperpolarization, but at a Vm more negative than -80 or -85 mV they decreased in amplitude. No frequency alterations were observed with these Vm changes. 4. The waxing-and-waning pattern of spontaneous spindles under barbiturate anaesthesia was distinct from the waning pattern under ketamine-xylazine anaesthesia. Under all anaesthetics, spindles had a waning pattern when elicited by cortical stimuli. The amplitude of cortical-evoked spindle waves diminished with the decrease in stimulation intensity. 5. Under urethane or ketamine-xylazine anaesthesia, spindle sequences were grouped by a cortically generated slow oscillation (< 1 Hz) and were preceded by a depth-positive EEG wave that corresponded to a prolonged hyperpolarization in all three investigated (cortical, thalamic reticular, and thalamocortical) cellular types. 6. We propose that the waxing pattern of spindle oscillation is due to a progressive entrainment of units into the oscillation until a maximum number is reached, depending on the background activity in the network. The phase relations between cortical, thalamic reticular and thalamocortical neurons are ascribed to distributed excitatory signals from thalamocortical neurons to both cortical and reticular neurons at each cycle of the oscillation. In turn, cortical neurons provide a powerful drive to potentiate the genesis of thalamic spindles.
Full text
PDF


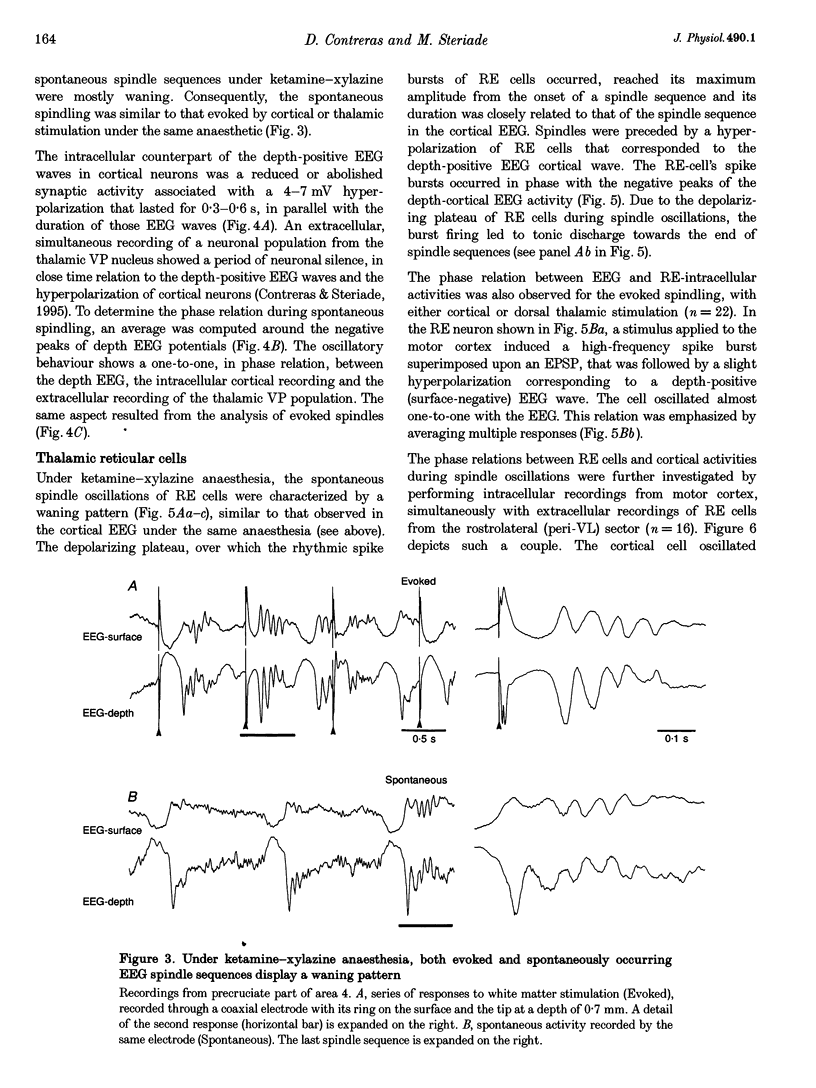
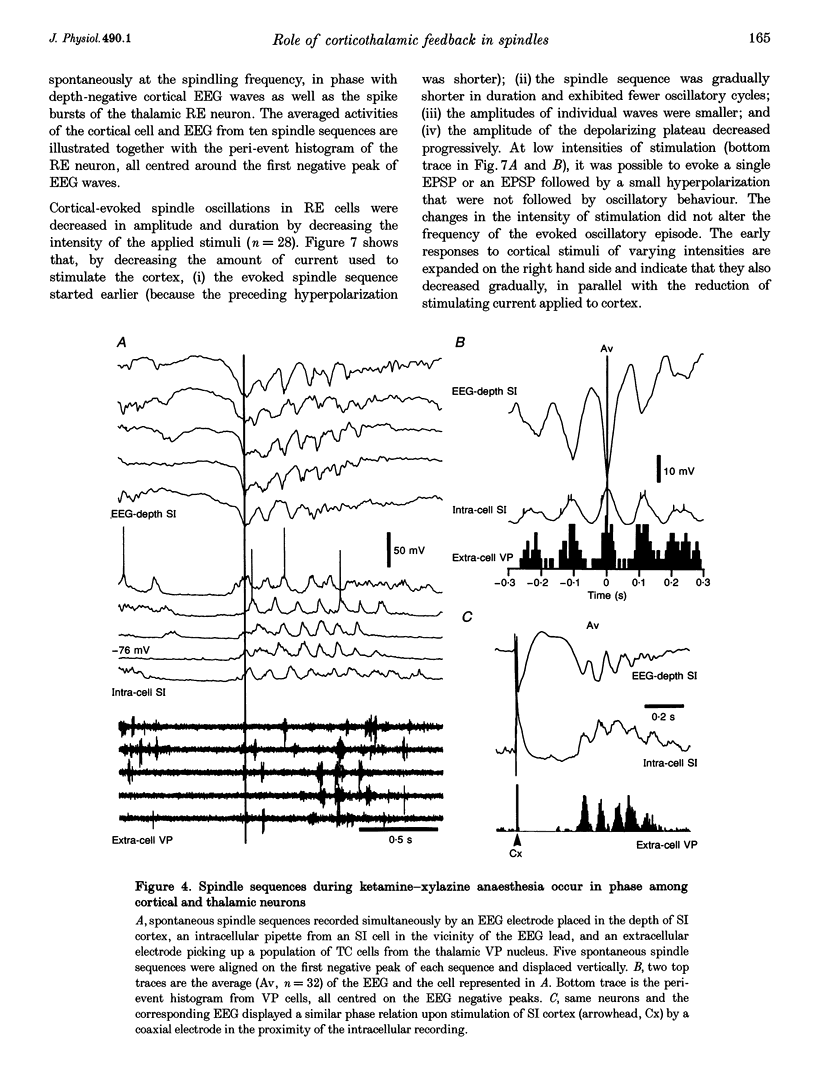





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzica F., Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol. 1995 Jan;73(1):20–38. doi: 10.1152/jn.1995.73.1.20. [DOI] [PubMed] [Google Scholar]
- Avanzini G., de Curtis M., Panzica F., Spreafico R. Intrinsic properties of nucleus reticularis thalami neurones of the rat studied in vitro. J Physiol. 1989 Sep;416:111–122. doi: 10.1113/jphysiol.1989.sp017752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M., Gloor P., Kostopoulos G., Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983 Oct;50(4):819–837. doi: 10.1152/jn.1983.50.4.819. [DOI] [PubMed] [Google Scholar]
- Bal T., McCormick D. A. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993 Aug;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T., von Krosigk M., McCormick D. A. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol. 1995 Mar 15;483(Pt 3):665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T., von Krosigk M., McCormick D. A. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995 Mar 15;483(Pt 3):641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D., Curró Dossi R., Steriade M. Electrophysiological properties of cat reticular thalamic neurones in vivo. J Physiol. 1993 Oct;470:273–294. doi: 10.1113/jphysiol.1993.sp019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D., Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995 Jan;15(1 Pt 2):604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A., Contreras D., Sejnowski T. J., Steriade M. A model of spindle rhythmicity in the isolated thalamic reticular nucleus. J Neurophysiol. 1994 Aug;72(2):803–818. doi: 10.1152/jn.1994.72.2.803. [DOI] [PubMed] [Google Scholar]
- Destexhe A., Contreras D., Sejnowski T. J., Steriade M. Modeling the control of reticular thalamic oscillations by neuromodulators. Neuroreport. 1994 Nov 21;5(17):2217–2220. doi: 10.1097/00001756-199411000-00003. [DOI] [PubMed] [Google Scholar]
- Domich L., Oakson G., Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J Physiol. 1986 Oct;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb D., Wang X. J., Rinzel J. Synchronization properties of spindle oscillations in a thalamic reticular nucleus model. J Neurophysiol. 1994 Sep;72(3):1109–1126. doi: 10.1152/jn.1994.72.3.1109. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Prince D. A. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992 Oct;12(10):3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Prince D. A. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994 Sep;14(9):5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982 Jun 3;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Amzica F. Dynamic coupling among neocortical neurons during evoked and spontaneous spike-wave seizure activity. J Neurophysiol. 1994 Nov;72(5):2051–2069. doi: 10.1152/jn.1994.72.5.2051. [DOI] [PubMed] [Google Scholar]
- Steriade M., Contreras D., Curró Dossi R., Nuñez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993 Aug;13(8):3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci. 1995 Jan;15(1 Pt 2):623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G., Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987 Jan;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986 Jan;6(1):68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Nuñez A., Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993 Aug;13(8):3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C. T., Jones E. G. Intracellular staining of physiologically identified neurons and axons in the somatosensory thalamus of the cat. Brain Res. 1983 Nov 28;280(1):148–154. doi: 10.1016/0006-8993(83)91183-6. [DOI] [PubMed] [Google Scholar]
- von Krosigk M., Bal T., McCormick D. A. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993 Jul 16;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]


