Abstract
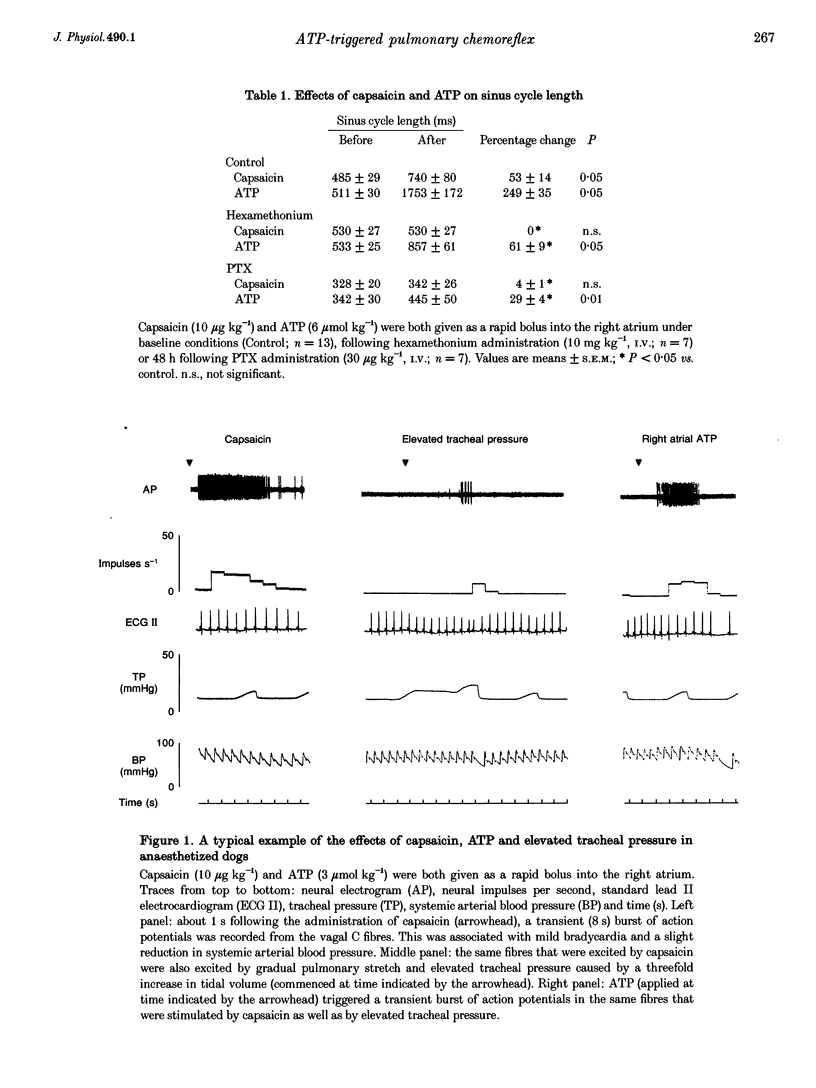
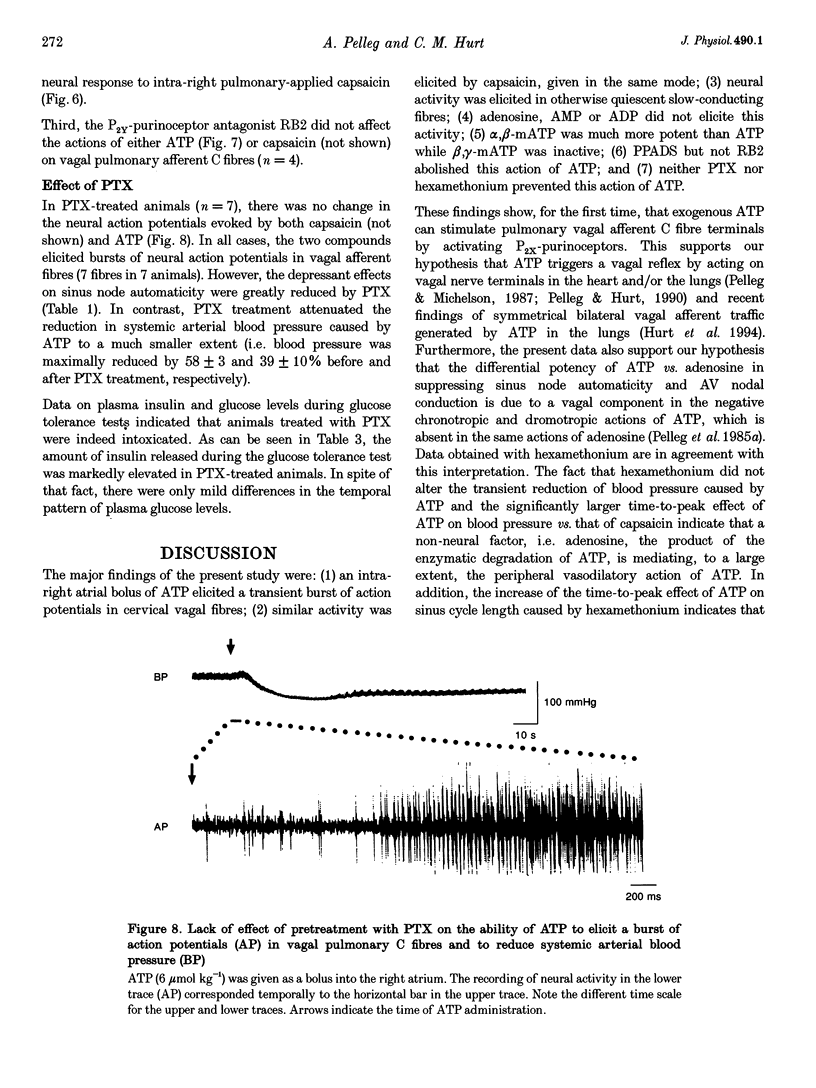
1. The effects of extracellular adenosine 5'-triphosphate (ATP) on pulmonary vagal afferent fibres (n = 46) was studied in a canine model in vivo (n = 38). 2. ATP (3-6 mumol kg-1), administered as a rapid bolus into the right atrium, elicited a transient burst of action potentials in cervical vagal fibres, which was not affected by either blockade of ganglionic transmission (hexamethonium) or a drop in arterial blood pressure (nitroglycerine). 3. The fibres with ATP-sensitive terminals were otherwise quiescent with no activity related to either cardiac or respiratory cycles and their conduction velocity was 0.85 +/- 0.13 m s-1 (n = 7). 4. Inflation of the lungs to 2-3 times the tidal volume triggered brief bursts of action potentials in these fibres. 5. Capsaicin (10 micrograms kg-1), given as a rapid bolus into the right atrium, elicited a burst of action potentials in these ATP-sensitive fibres. 6. Smaller amounts of ATP and capsaicin (0.5-3 mumol kg-1 and 1-5 micrograms kg-1, respectively) had similar effects when the two compounds were given into the right pulmonary artery. 7. Adenosine, adenosine 5'-monophosphate, or adenosine 5'-diphosphate did not excite these fibres (n = 30). 8. The non-degradable analogue of ATP alpha,beta-methylene ATP (alpha,beta-mATP) was tenfold more potent than ATP while beta,gamma-methylene ATP (beta,gamma-mATP) was in active. 9. The selective P2x-purinoceptor antagonist pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid markedly attenuated the effect of ATP but not of capsaicin. The P2Y-purinoceptor antagonist Reactive Blue 2 was without effect. 10. Pretreatment with pertussis toxin (PTX) did not affect this action of ATP. 11. In the canine lungs ATP activates vagal C fibre nerve terminals. This action is mediated by P2X-purinoceptors and is independent of a PTX-sensitive guanine nucleotide binding protein (G protein).
Full text
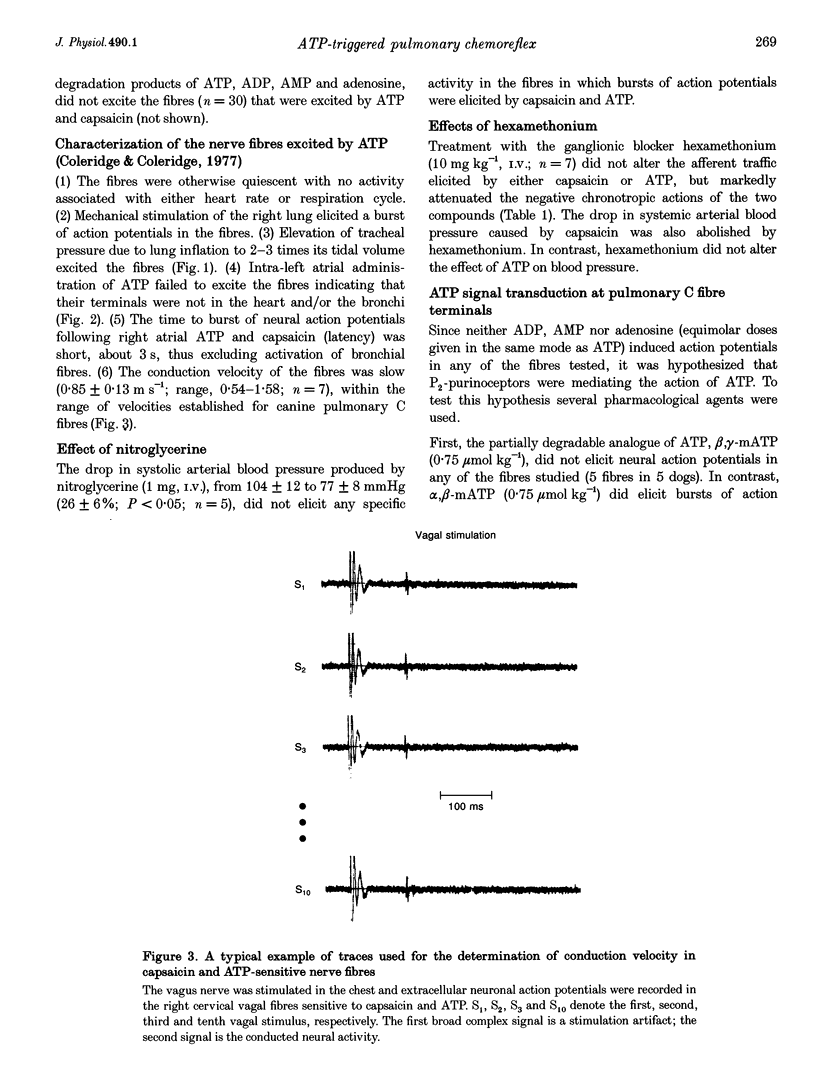
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour J. A., Huang M. H., Pelleg A., Sylvén C. Responsiveness of in situ canine nodose ganglion afferent neurones to epicardial mechanical or chemical stimuli. Cardiovasc Res. 1994 Aug;28(8):1218–1225. doi: 10.1093/cvr/28.8.1218. [DOI] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
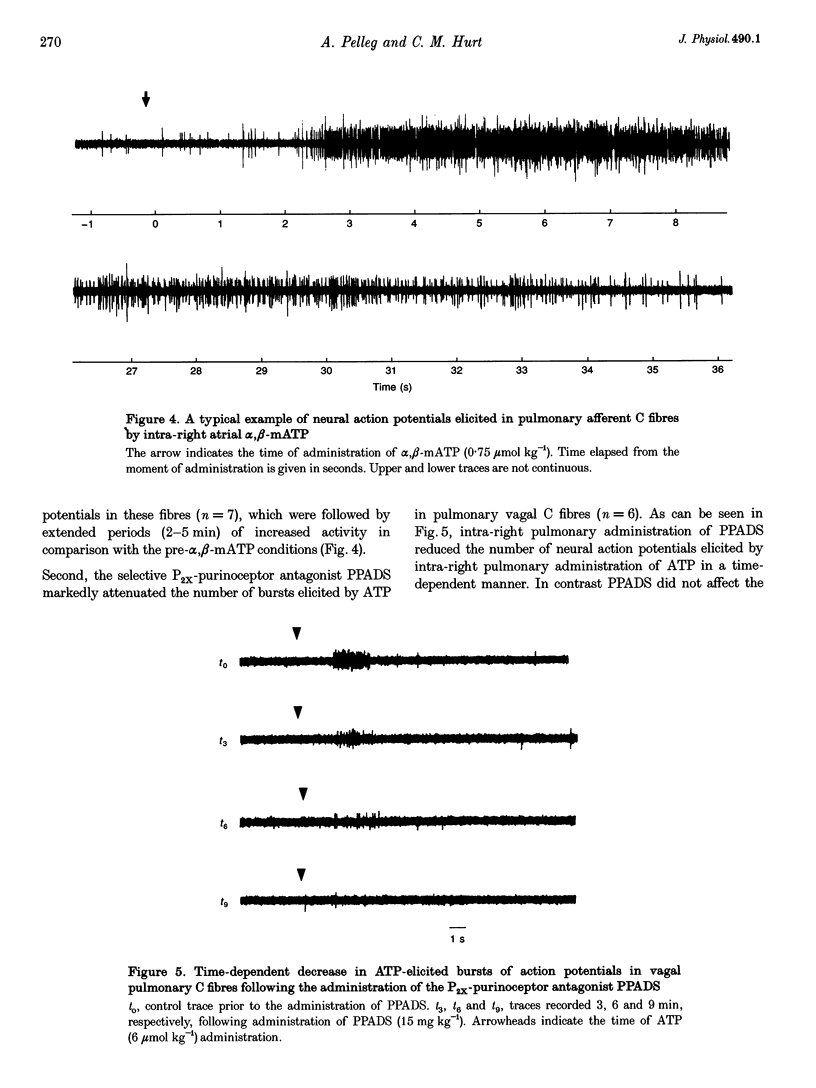
- Bean B. P., Friel D. D. ATP-activated channels in excitable cells. Ion Channels. 1990;2:169–203. doi: 10.1007/978-1-4615-7305-0_5. [DOI] [PubMed] [Google Scholar]
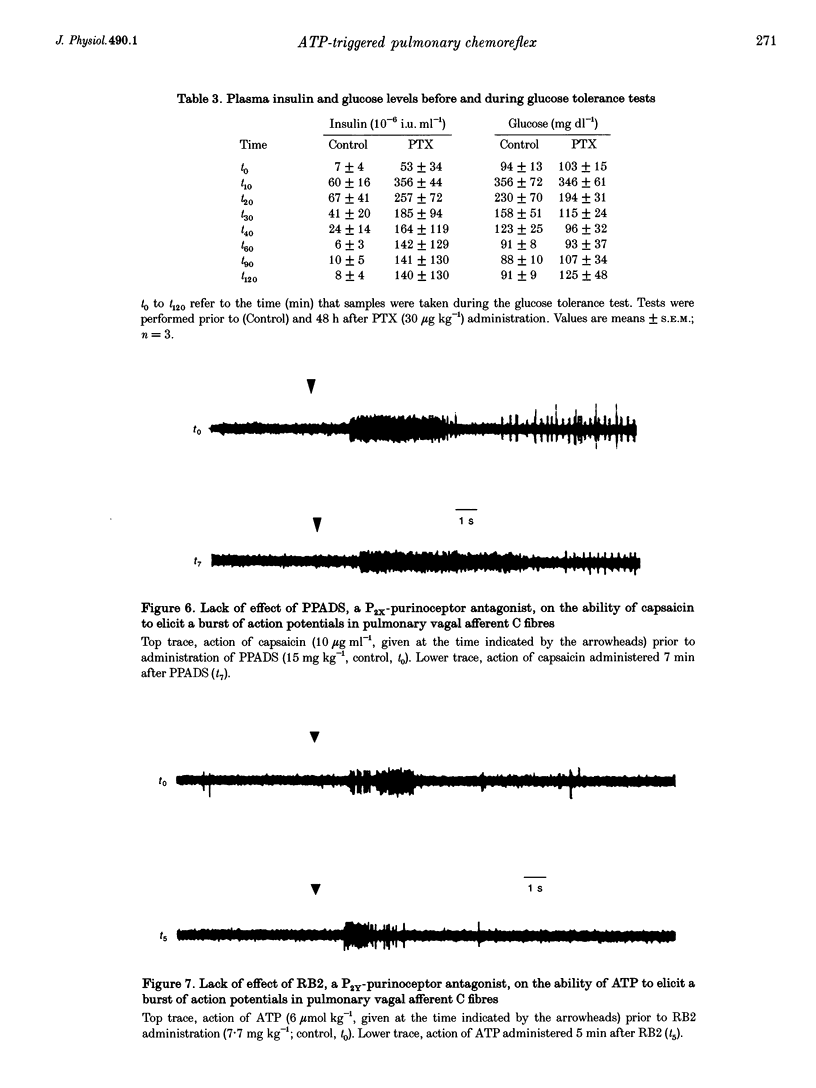
- Benham C. D. Neurotransmitters. ATP joins the fast lane. Nature. 1992 Sep 10;359(6391):103–104. doi: 10.1038/359103a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976 Aug;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- CARDENAS M., ACEVES J., ALARCON G. EFFECTO DEL 'ACIDO ADENOSINTRIFOSF'ORICO SOBRE LAS PROPIEDADES FISIOL'OGICAS DEL CORAZ'ON. Arch Inst Cardiol Mex. 1964 Jul-Aug;34:485–494. [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C. Impulse activity in afferent vagal C-fibres with endings in the intrapulmonary airways of dogs. Respir Physiol. 1977 Apr;29(2):125–142. doi: 10.1016/0034-5687(77)90086-x. [DOI] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Luck J. C. Pulmonary afferent fibres of small diameter stimulated by capsaicin and by hyperinflation of the lungs. J Physiol. 1965 Jul;179(2):248–262. doi: 10.1113/jphysiol.1965.sp007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., COMROE J. H., Jr Chemoreflexes from the heart and lungs. Physiol Rev. 1954 Apr;34(2):167–201. doi: 10.1152/physrev.1954.34.2.167. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe R. E., Perl E. R. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proc Natl Acad Sci U S A. 1984 Nov;81(21):6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. H., Sylvén C., Pelleg A., Smith F. M., Armour J. A. Modulation of in situ canine intrinsic cardiac neuronal activity by locally applied adenosine, ATP, or analogues. Am J Physiol. 1993 Oct;265(4 Pt 2):R914–R922. doi: 10.1152/ajpregu.1993.265.4.R914. [DOI] [PubMed] [Google Scholar]
- Hurt C. M., Wang L., Xu J., Sterious W., Pelleg A. Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in dogs. II. Vagal afferent traffic. Am J Physiol. 1994 Sep;267(3 Pt 2):H1093–H1097. doi: 10.1152/ajpheart.1994.267.3.H1093. [DOI] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G., Volkova T. M. Receptors for ATP in rat sensory neurones: the structure-function relationship for ligands. Br J Pharmacol. 1988 Dec;95(4):1057–1062. doi: 10.1111/j.1476-5381.1988.tb11739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht G., Friebe T., Grimm U., Windscheif U., Bungardt E., Hildebrandt C., Bäumert H. G., Spatz-Kümbel G., Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur J Pharmacol. 1992 Jul 7;217(2-3):217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Robb I. A., Roberts G. C. The release of nucleotides, 5-hydroxytryptamine and enzymes from human blood platelets during aggregation. J Physiol. 1968 Apr;195(3):715–729. doi: 10.1113/jphysiol.1968.sp008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- Pelleg A., Belhassen B., Ilia R., Laniado S. Comparative electrophysiologic effects of adenosine triphosphate and adenosine in the canine heart: influence of atropine, propranolol, vagotomy, dipyridamole and aminophylline. Am J Cardiol. 1985 Feb 15;55(5):571–576. doi: 10.1016/0002-9149(85)90249-8. [DOI] [PubMed] [Google Scholar]
- Pelleg A., Hurt C. M., Soler-Baillo J. M., Polansky M. Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in dogs. Am J Physiol. 1993 Aug;265(2 Pt 2):H681–H690. doi: 10.1152/ajpheart.1993.265.2.H681. [DOI] [PubMed] [Google Scholar]
- Pelleg A., Mitamura H., Michelson E. L., Dreifus L. S. Evidence against prostaglandin mediation of the differential electrophysiologic effects of ATP versus adenosine in the canine heart. J Cardiovasc Pharmacol. 1986 May-Jun;8(3):534–538. doi: 10.1097/00005344-198605000-00015. [DOI] [PubMed] [Google Scholar]
- Pelleg A., Mitamura H., Michelson E. L. Evidence for vagal involvement in the electrophysiologic actions of exogenous adenosine and adenosine triphosphate in the canine heart. J Auton Pharmacol. 1985 Sep;5(3):207–212. doi: 10.1111/j.1474-8673.1985.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Ronca-Testoni S., Borghini F. Degradation of perfused adenine compounds up to uric acid in isolated rat heart. J Mol Cell Cardiol. 1982 Mar;14(3):177–180. doi: 10.1016/0022-2828(82)90116-x. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Hubbard J. I. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature. 1973 Jun 15;243(5407):404–405. doi: 10.1038/243404a0. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol Sci. 1991 Sep;12(9):319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]


