ABSTRACT
Background and Aims
Chronic refractory wound is a disease that seriously impairs the quality of life of patients. Negative pressure wound therapy and platelet‐rich plasma are commonly used to treat various types of wounds. Further research is necessary to explore the efficacy and safety of the combination of negative pressure wound therapy and platelet‐rich plasma in treating chronic refractory wounds.
Methods
PubMed, Web of Science, EMBASE, Cochrane, CINAHL, CNKI, Sino Med, and Wanfang Med Online up until March 2024 were searched(PROSPERO No. CRD42024507963). Two investigators screened literature according to inclusion and exclusion criteria, evaluated bias and certainty of evidence using RoB 2.0 and GRADE. Stata 12.0 was used to analyze the data.
Results
A total of 35 randomized controlled trials involving 2495 participants were included. 34 studies were assessed as having some concerns, and 1 study as having high risk in the risk of bias assessment. The results of meta‐analysis showed that effective rate (RR1.23, 95% CI [1.17, 1.30], p < 0.001; I2 = 44.7%, p = 0.013), healing time (WMD‐9.32, 95% CI [−10.60, −8.03], p < 0.001; I² = 91.00%, p < 0.001), healing rate (RR1.76, 95% CI [1.50, 2.07], p < 0.001; I2 = 62.6%, p < 0.001), positive rate of bacterial(RR0.25, 95% CI [0.15, 0.40], p < 0.001; I² = 0%, p = 0.841), pain score (WMD‐1.43, 95% CI [−2.14, −0.72], p < 0.001; I² = 96.5%, p < 0.001), incidence of complications (RR0.45, 95% CI [0.30, 0.68], p < 0.001; I² = 46.3%, p = 0.098), length of hospital stay (WMD‐9.88, 95% CI [−13.42, 6.34], p < 0.001; I2 = 98.9%, p < 0.001), number of dressing changes (WMD‐2.56, 95% CI [−4.28, −0.83], p = 0.004; I² = 98.9%, p < 0.001), white blood cell level (WMD‐1.71, 95% CI [−2.00, −1.41], p < 0.001; I² = 33.9%, p = 0.195), c‐reactive protein level (WMD‐0.68, 95% CI [−1.04, −0.33], p < 0.001; I² = 88.8%, p < 0.001), erythrocyte sedimentation rate (WMD‐6.09, 95% CI [−8.05, −4.13], p < 0.001; I² = 13%, p = 0.32), score of vancouver scar scale (WMD‐1.78, 95% CI [−1.89, −1.66], p < 0.001; I² = 38.3%, p = 0.166) and preparation time of secondary repair (WMD‐4.95, 95% CI [−7.03, −2.87], p < 0.001; I² = 84.7%, p < 0.001) had statistically significant effects. However, hospitalization costs (WMD1423.56, 95% CI [−4588.93, 7436.06], p = 0.643; I2 = 100%, p < 0.001) had no significant difference.
Conclusions
This study demonstrates that the combination of negative‐pressure wound therapy and platelet‐rich plasma can improve the efficacy and safety on chronic refractory wounds. Optimal parameter combinations, elucidation of pathogenesis and treatment mechanisms can be explored in the future.
Keywords: chronic refractory wound, meta‐analysis, negative‐pressure wound therapy, platelet‐rich plasma, wound healing, wounds and injuries
1. Introduction
Chronic refractory wounds (CRWs) are affected by various factors [1, 2, 3, 4], which are defined as wounds that fail to heal for more than 1 month and have no tendency to heal in clinical practice. CRWs is characterized by a prolonged inflammatory response period, persistence of infection, and failure of epidermal and dermal cells to respond [5]. Common causes of CRWs include infection, diabetes, pressure ulcers, trauma, arterial and venous ulcers, et al. There are about 4.5 million CRWs patients in the United States each year, and the cost of wound care is about 28 to 96.8 billion dollars [6]. In Australia, CRWs‐related medical costs exceed AUD 3.5 billion, accounting for about 2% [1]. In China, the number of people who need wound treatment is about 100 million per year, of which CRWs is as high as 30 million [1]. Due to the long course and great harm, how to improve the speed and quality of wound healing has always been a hot topic in clinical research.
Negative pressure wound therapy (NPWT) is a commonly used debridement and drainage method to transform passive drainage into active suction drainage [7]. It can convert an open wound to a closed wound for intermittent or continuous suction. In the international consensus guidelines, NPWT is recommended to be applied to all kinds of infected and refractory wounds [8]. The treatment goal of NPWT is to treat and protect the CRWs, and provide clean wound bed preparation [7]. However, NPWT does not have the characteristics of biological agents providing inflammatory cells and growth factors to promote self‐healing of wounds.
Mobilization of its own growth factors can improve active repair of wounds. However, the local environment of CRWs has a low number and activity of growth factors. Platelet‐rich plasma (PRP) contains an abundance of various cytokines that promote tissue regeneration and facilitate repair [9]. The clinical efficacy of PRP has been well established through its widespread use in various medical specialties, including orthopedics, stomatology, ophthalmology, obstetrics and gynecology, plastic and reconstructive surgery [10, 11, 12, 13, 14].
The combination of NPWT and PRP holds promising prospects in the field of regenerative medicine. However, there is the absence of standardized guidelines and recommendations for utilizing NPWT combined with PRP. Meanwhile, there is inconsistency in therapeutic outcomes among patients with CRWs. Although Chen's latest research [15] has already published the results on the efficacy of NPWT combined with PRP, this study incorporated a more extensive studies and provided comprehensive support indicators, including efficacy indicators, safety indicators and cost‐effectiveness indicators. Thus, this study aims to conduct a meta‐analysis by searching relevant randomized controlled clinical trials (RCTs) to analyze the efficacy and safety of NPWT combined with PRP, furnishing robust evidence for further comprehensive exploration of its efficacy and mechanism.
2. Data and Methods
The process and results of this systematic review were described and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyzes (PRISMA) guidelines [16] and the Cochrane Handbook for Systematic Reviews of interventions. The protocol for this systematic review has been pre‐registered on PROSPERO (CRD42024507963).
2.1. Literature Search
A comprehensive computerized search of PubMed, Web of Science, EMBASE, CINAHL, CENTRAL, CNKI (For Chinese), Sino Med (For Chinese), Wanfang Med Online (For Chinese) were performed from inception to March 2024. For a further comprehensive search, we obtained more comprehensive data by consulting the reference lists of included articles and relevant conference papers, and contacting the authors of potentially ongoing or unpublished studies in the field. The complete search strategy is provided in the Appendix S1.
2.2. Inclusion and Exclusion Criteria
The articles included in the systematic review had to meet the following requirements: (1) Population: patients aged 18–65 years with CRWs (including chronic wounds, refractory wounds, pressure injuries, diabetic foot, vascular ulcers, et al.); (2) Intervention: use of any type of NPWT combined with any product containing PRP; (3) Comparison: use of NPWT alone, common care, no intervention or other alternative treatment; (4) Outcomes: use of a reliable assessment method [effective rate, healing time, healing rate, length of hospital stay, number of dressing changes, hospital costs, positive rate of bacterial, white blood cell level (WBC), c‐reactive protein level (CRP), erythrocyte sedimentation rate level (ESR), pain score, vancouver scar scale (VSS), incidence of complications, preparation time for secondary repair] to evaluate the efficacy and safety of CRWs. (5) Study of design: only RCTs published in Chinese or English were included.
The articles will be excluded if they met the following criteria: (1) studies with incomplete or unclear analysis data and inconsistent outcome indicators; (2) articles with poor research quality and lack of original data.
2.3. Data Extraction
Two researchers independently screened the literature search results using NoteExpress V3.0 software. Two researchers used a predesigned data extraction table to extract relevant information from the included studies, including authors and publication years, characteristics of included studies, participants, interventions, and outcomes. Any disagreements between the two researchers during the cross‐validation process on literature screening and information extraction were resolved by discussion with the third researcher. If there were missing data and information in the included articles, we contacted the authors to obtain relevant content to promote the accuracy of the analysis. For articles only provide chart data, using the image data extraction tool (https://apps.automeris.io/wpd/index.zh_CN.html) for processing.
2.4. Quality Assessment
2.4.1. Risk of Bias
The Cochrane Risk of Bias 2.0 tool (2019 revision) of the Cochrane Collaboration was used to assess the quality of RCTs [17]. The tool assessed five domains of bias: randomization process, deviations from the intended intervention, missing outcome data, measurement of outcome, and selection of reporting result. Each domain contained a number of questions that were judged as “yes (Y),” “probably yes (PY),” “no (N),” “probably no (PN),” and “no information(NI)” based on the included study. “Low,” “some concern,” and “high” results were obtained based on the above questions, which were used to classify risk of bias judgments. Two researchers used “with macro Excel tool”(https://www.riskofbias.info/) independently evaluated. Cross‐checking was performed after the evaluation, and disagreements that emerged were resolved by discussion with the third researcher.
2.4.2. Quality Assessment of Evidence
GRADE (Grading of Recommendation, Assessment, Development, and Evaluation) provided clear criteria to evaluate the certainty [18, 19]. This study used GRADE to assess the quality of the certainty of evidence for each outcome. The quality of evidence for each outcome was rated as high, moderate, low or very low through considering risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of RCTs was initially considered high and then downgraded [20, 21, 22, 23, 24].
2.5. Data Analysis
Stata12.0 software was used for meta‐analysis. Risk Ratio (RR) with 95% confidence interval (CI) was used for the count data, and weighted mean difference (WMD) with 95% CI was used for the measurement data. Two‐tailed p‐value < 0.05 was considered to indicate statistical significance. Chi‐square tests (χ2) and inconsistency (I2) were used to calculate statistical heterogeneity. When heterogeneity was statistically significant (p ≤ 0.10, I2 > 50%), the data were pooled and analyzed using a random‐effect model. Instead, a fixed‐effect model (p > 0.10, I2 ≤ 50%) was applied. The source of heterogeneity was explored by subgroup analysis. Sensitivity analysis was used to assess the robustness of the results by excluding studies one by one. If the number of included studies was ≥ 10, funnel plots as well as Begg's and Egger's tests were performed to assess publication bias.
3. Results
3.1. Study Description
The initial search of the database yielded a total of 1102 studies, of which 431 were removed due to duplication. After careful review of titles and abstracts, 612 articles were excluded. The remaining 59 articles were read in full, 11 articles could not be obtained in full text, and 13 articles were deemed unsuitable for further analysis (Appendix S2). Finally, a total of 35 articles were included [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59]. The literature screening process and results are shown in Figure 1. The 35 articles covered 2495 patients diagnosed with CRWs. The characteristics of the studies involved are summarized in Table 1, more details are showed in Appendix S3. The studies were published between 2015 and 2022, of which 26 (74.29%) were published after 2019. All studies used RCT design. All the intervention groups used NPWT combined with PRP to treat CRWs. The control group in 4 studies [27, 29, 33, 53] used common care (CC), and the remaining 31 used a single NPWT for CRWs. 6 studies [25, 35, 48, 50, 52, 57] were supported by government academic funds, and 6 studies [36, 43, 50, 57, 58, 59] reported conflicts of interest.
Figure 1.
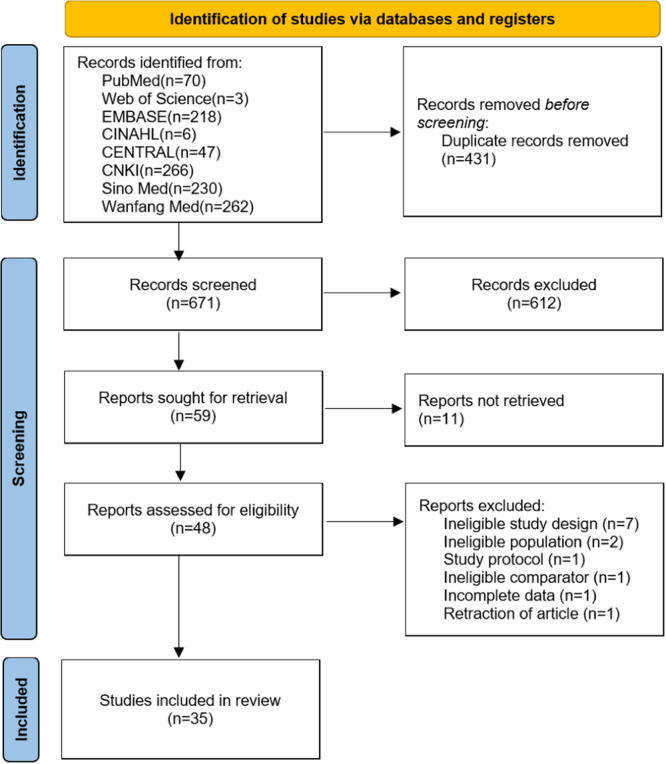
Flowchart showing the study selection process.
Table 1.
Characteristics of the included studies.
| Author | Year | Type | Sample size | Gender (male/female) | Age (years) | |||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | |||
| Biao | 2019 | RCT | 25 | 25 | 19/6 | 17/8 | 61.04 ± 11.869 | 59.04 ± 12.212 |
| Fei | 2020 | RCT | 20 | 20 | 12/8 | 11/9 | 53.1 ± 9.12 | 54.1 ± 10.04 |
| Fuzeng | 2021 | RCT | 60 | 60 | 34/26 | 31/29 | 43.24 ± 2.52 | 42.85 ± 2.33 |
| Jinhu | 2018 | RCT | 22 | 22 | 12/10 | 11/11 | 71.59 ± 6.57 | 71.91 ± 6.71 |
| Juan | 2019 | RCT | 30 | 30 | 19/11 | 20/10 | 62.50 ± 10.50 | 62.23 ± 12.43 |
| Li | 2021 | RCT | 50 | 50 | 29/21 | 27/23 | 61.62 ± 8.35 | 60.88 ± 7.98 |
| Lihua | 2017 | RCT | 36 | 36 | 21/15 | 19/17 | 64.6 ± 13.8 | 66.5 ± 16.2 |
| Ling | 2022 | RCT | 24 | 24 | 14/10 | 18/6 | 58.17 ± 13.08 | 55.50 ± 15.36 |
| Qian | 2021 | RCT | 51 | 51 | 25/26 | 27/24 | 60.79 ± 6.38 | 59.36 ± 6.21 |
| Rao | 2020 | RCT | 15 | 15 | 11/4 | 10/5 | 43.0 ± 6.1 | 42.1 ± 5.6 |
| Siwen | 2021 | RCT | 20 | 20 | 12/8 | 12/8 | 58.8 ± 13.82 | 52.4 ± 15.80 |
| Wenhua | 2021 | RCT | 49 | 49 | 30/19 | 29/20 | 60.7 ± 6.9 | 59.2 ± 6.7 |
| Xuecheng | 2017 | RCT | 43 | 44 | 29/14 | 30/14 | 64.0 ± 1.5 | 63.0 ± 2.1 |
| Zhongxing | 2022 | RCT | 46 | 45 | 31/15 | 34/11 | 56.15 ± 12.23 | 54.37 ± 11.89 |
| Changzhu | 2018 | RCT | 34 | 34 | 19/15 | 18/16 | 62.40 ± 0.3 | 61.70 ± 0.40 |
| Chijiao | 2018 | RCT | 42 | 42 | 29/13 | 28/14 | 54 ± 12 | 54 ± 12 |
| Feifei | 2020 | RCT | 30 | 30 | 16/14 | 17/13 | 62.2 ± 2.4 | 61.3 ± 2.5 |
| Guanlong | 2022 | RCT | 39 | 39 | 27/12 | 21/18 | 58.74 ± 5.19 | 60.20 ± 4.83 |
| Guoguang | 2021 | RCT | 32 | 32 | 16/16 | 18/14 | 48.82 ± 2.73 | 48.97 ± 2.75 |
| Guoyang | 2021 | RCT | 55 | 55 | 28/27 | 27/28 | 53.25 ± 9.56 | 54.66 ± 8.79 |
| Hongwei | 2019 | RCT | 32 | 32 | 18/14 | 19/13 | 64.46 ± 5.46 | 65.06 ± 5.50 |
| Jinpeng | 2018 | RCT | 36 | 36 | 24/12 | 25/11 | 19‐45 | 18‐46 |
| Jixiang | 2015 | RCT | 39 | 42 | 30/9 | 32/10 | 53.87 ± 5.76 | 54.13 ± 5.82 |
| Maisvuti | 2021 | RCT | 37 | 37 | 23/14 | 20/17 | 63.6 ± 10.2 | 63.3 ± 10.5 |
| Peng | 2019 | RCT | 47 | 47 | 25/22 | 27/20 | 55.33 ± 6.58 | 56.35 ± 6.39 |
| Qingjian | 2020 | RCT | 31 | 31 | 21/10 | 20/11 | 48.26 ± 6.57 | 47.85 ± 6.47 |
| Quan | 2021 | RCT | 39 | 39 | 21/18 | 19/20 | 52.21 ± 5.34 | 52.51 ± 5.23 |
| Qunfang | 2020 | RCT | 30 | 30 | Unknown | Unknown | Unknown | Unknown |
| Rilun | 2020 | RCT | 49 | 49 | 26/23 | 25/24 | 61.89 ± 15.02 | 62.03 ± 14.78 |
| Ronghua | 2020 | RCT | 30 | 30 | 18/12 | 17/13 | 42.16 ± 2.12 | 42.35 ± 2.18 |
| Xinchan | 2018 | RCT | 28 | 30 | 13/15 | 12/18 | 49.54 ± 1.55 | 49.59 ± 1.54 |
| Xionghua | 2021 | RCT | 32 | 32 | 16/16 | 15/17 | 56.5 ± 23.5 | 55.5 ± 24.5 |
| Yaping | 2019 | RCT | 21 | 21 | 23/19 | Unknown | 48.63 ± 3.32 | 48.63 ± 3.32 |
| Zhenqiang | 2021 | RCT | 20 | 20 | 13/7 | 12/8 | 5–70 | 7–70 |
| Xin | 2022 | RCT | 68 | 34 | 14/20 | 16/18 | 48.91 ± 7.92 | 48.87 ± 7.88 |
3.2. Risk of Bias Assessment
The risk of bias assessment of the included studies was conducted across multiple domains (Figures 2 and 3). 34 studies were assessed as having some concerns, and 1 study as having high risk. A total of 35 studies reported the use of randomization, but 11 studies [27, 31, 32, 34, 39, 40, 44, 48, 51, 52, 54] did not report the specific randomization methods. All studies did not report concealment of allocation sequence before participants enrolled and assigned to interventions. The randomization process of all studies had some concerns, except for 1 study [38] which was assessed as high risk due to the lack of reporting baseline differences between intervention groups. In addition, all the studies were not blinded to participants and care givers in assessing deviations from intended interventions. However, blinding is difficult due to the specificity of the intervention. Thus, all the studies were assessed as having some concerns in the domain of deviations from intended interventions. All the studies were assessed as having low risk in the domain of missing outcome data because they had complete outcomes and no missing data. Although none of the 35 studies were blinded to the intervention, these 23 studies [25, 27, 28, 29, 30, 31, 32, 33, 36, 37, 38, 39, 40, 42, 44, 45, 46, 48, 51, 53, 54, 55, 56] did not involve subjective measures and were therefore rated as having low risk in the domain of outcome measurement, and the remaining 12 studies [26, 34, 35, 41, 43, 47, 49, 50, 52, 57, 58, 59] were assessed as having some concerns because they involved assessment of pain which related to subjective consciousness judgment. In the domain of selection of reported result, all the studies were assessed as having some concerns because they did not mention study protocol registration information and could not be accessed.
Figure 2.
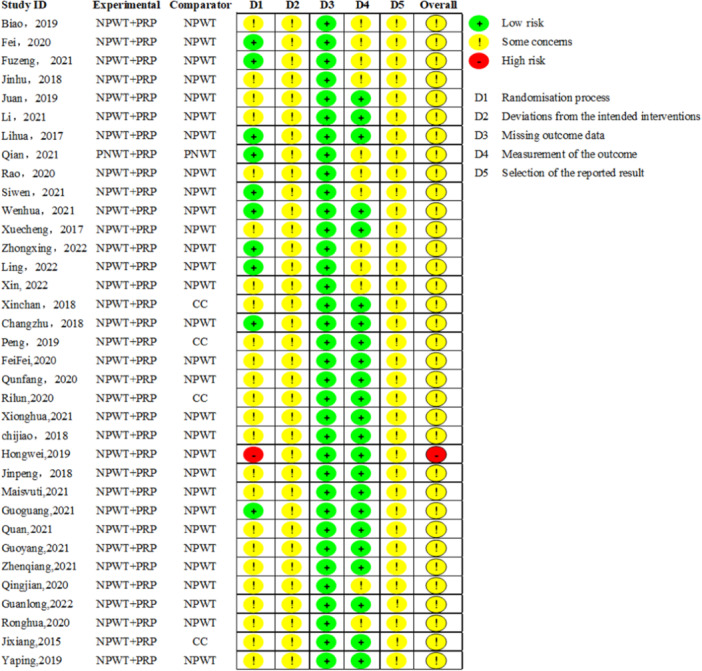
Summary plot of the risk of bias of the included studies.
Figure 3.
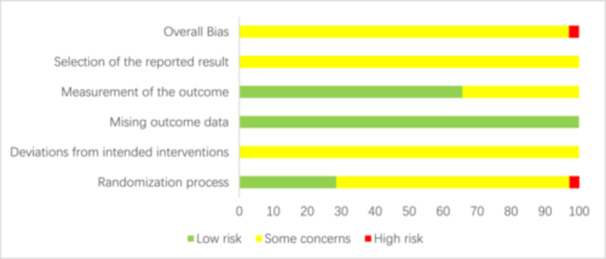
Chart of the percentage risk of bias of the included literature.
3.3. Data Synthesis
Full meta‐analysis results can be found in Table 2 and Appendix 4. Effective rate (RR1.23, 95% CI [1.17, 1.30], p < 0.001), healing rate (RR1.76, 95% CI [1.50, 2.07], p < 0.001), healing time (WMD‐9.32, 95% CI [−10.60, −8.03], p < 0.001), preparation time of secondary repair (WMD‐4.95, 95% CI [−7.03, −2.87], p < 0.001), score of vancouver scar scale (WMD‐1.78, 95% CI [−1.89, −1.66], p < 0.001), positive rate of bacterial (RR0.25, 95% CI [0.15, 0.40], p < 0.001), CRP level (WMD‐0.68, 95% CI [−1.04, −0.33], p < 0.001), ESR level (WMD‐6.09, 95% CI [−8.05, −4.13], p < 0.001), WBC level (WMD‐1.71, 95% CI [−2.00, −1.41], p < 0.001), pain score (WMD‐1.43, 95% CI [−2.14, −0.72], p < 0.001), length of hospital stay (WMD‐9.88, 95% CI [−13.42, −6.34], p < 0.001), number of dressing changes (WMD‐2.56, 95% CI [−4.28, −0.83], p = 0.004) and incidence of complications (RR0.45, 95% CI [0.30, 0.68], p < 0.001) were better in NPWT combined with PRP groups versus NPWT or common care groups. Hospitalization costs had no significant difference.
Table 2.
Full meta‐analysis results.
| Outcomes (Number of studies) | Number of participants | Effects | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Int. | Com. | Effect model | RR or WMD | 95% CI | P value | I2 | P value | ||
| Primary outcomes | Effective rate (22) | 1648 | 823 | 825 | Random | RR1.23 | [1.17, 1.30] | p < 0.001 | 44.7% | p = 0.013 |
| Healing time (27) | 1947 | 971 | 976 | Random | WMD‐9.32 | [−10.60, −8.03] | p < 0.001 | 91.00% | p < 0.001 | |
| Healing rate (23) | 1630 | 812 | 818 | Random | RR1.76 | [1.50, 2.07] | p < 0.001 | 62.6% | p < 0.001 | |
| Hospitalization costs (7) | 548 | 273 | 275 | Random | WMD1423.56 | [−4588.93, 7436.06] | p = 0.643 | 100% | p < 0.001 | |
| Positive rate of bacterial (6) | 303 | 151 | 152 | Fixed | RR0.25 | [0.15, 0.40] | p < 0.001 | 0% | p = 0.841 | |
| Pain score, 14 d (9) | 474 | 237 | 237 | Random | WMD‐1.43 | [−2.14, −0.72] | p < 0.001 | 96.5% | p < 0.001 | |
| Incidence of complications (7) | 634 | 317 | 317 | Fixed | RR0.45 | [0.30, 0.68] | p < 0.001 | 46.3% | p = 0.098 | |
| Secondary outcomes | Length of Hospital stay (16) | 1185 | 591 | 594 | Random | WMD‐9.88 | [−13.42, −6.34] | p < 0.001 | 98.9% | p < 0.001 |
| Number of dressing changing (4) | 322 | 160 | 162 | Random | WMD‐2.56 | [−4.28, −0.83] | p = 0.004 | 98.9% | p < 0.001 | |
| White blood cell level, WBC (5) | 267 | 133 | 134 | Fixed | WMD‐1.71 | [−2.00, −1.41] | p < 0.001 | 33.9% | p = 0.195 | |
| C‐reactive protein level, CRP (7) | 436 | 218 | 218 | Random | WMD‐0.68 | [−1.04, −0.33] | p < 0.001 | 88.8% | p < 0.001 | |
| Erythrocyte sedimentation rate level, ESR (3) | 150 | 75 | 75 | Fixed | WMD‐6.09 | [−8.05, −4.13] | p < 0.001 | 13% | p = 0.32 | |
| Score of Vancouver scar scale, VSS (5) | 292 | 146 | 146 | Fixed | WMD‐1.78 | [−1.89, −1.66] | p < 0.001 | 38.3% | p = 0.166 | |
| Preparation time for secondary repair (6) | 346 | 173 | 173 | Random | WMD‐4.95 | [−7.03, −2.87] | p < 0.001 | 84.7% | p < 0.001 | |
Abbreviations: 95% CI, confidence interval; Com, Comparison group; Fixed, fixed‐effect model; I2, inconsistency; Int, Intervention group; Random, random‐effect model; RR, Risk Ratio; WMD, weighted mean difference.
3.4. Subgroup Analysis
The full subgroup analyzes are showed in Table 3 and Appendix S5. The results of subgroup analysis showed wound type, negative pressure type and mode of operation were not the sources of heterogeneity. Subgroup analysis revealed that the NPWT before PRP group exhibited higher effective rate, higher CRP level and a greater number of dressings changing compared to both the PRP before NPWT group and the simultaneous treatment group. The length of hospital stay was longer in the PRP before NPWT group. The healing rate of pressure injury group was lower compared to both mixed wound group and diabetic foot group, while hospitalization cost was higher for diabetic foot group compared to those with mixed wound group. Additionally, intermittent negative pressure group demonstrated better hospitalization costs than continuous negative pressure group or unknown negative pressure group, with unknown negative pressure group requiring a longer preparation time for secondary repair.
Table 3.
Full subgroup analysis results.
| Outcomes | Subgroup by | Studies, n | Effects | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| Effect size (WMD or RR) | 95% CI | P value | I2 (%) | P value | ||||
| Effective rate | Wound type | Mixed wound | 6 | 1.37 | 1.14, 1.65 | 0.001 | 56.2 | 0.008 |
| Diabetic foot | 14 | 1.21 | 1.14, 1.28 | < 0.001 | 28.3 | 0.067 | ||
| Pressure injury | 2 | 1.22 | 1.08, 1.37 | 0.001 | 0.0 | 0.888 | ||
| negative pressure type | Continuous nagative pressure | 9 | 1.18 | 1.11, 1.25 | < 0.001 | 11.7 | 0.338 | |
| Intermittent negative pressure | 2 | 1.38 | 1.15, 1.65 | < 0.001 | 0.0 | 0.981 | ||
| unknown | 11 | 1.27 | 1.15, 1.39 | < 0.001 | 58.7 | 0.007 | ||
| Mode of operation | NPWT before PRP | 18 | 1.24 | 1.17, 1.31 | < 0.001 | 36.3 | 0.063 | |
| PRP before NPWT | 2 | 1.11 | 1.00, 1.23 | 0.056 | 0.0 | 0.510 | ||
| Proceed simultaneously | 2 | 1.64 | 0.78, 3.47 | 0.193 | 86.6 | 0.006 | ||
| Healing time | Wound type | Mixed wound | 10 | −8.32 | −10.69, −5.94 | < 0.001 | 86.9 | < 0.001 |
| Diabetic foot | 15 | −10.61 | −12.17, −9.04 | < 0.001 | 90.2 | < 0.001 | ||
| Pressure injury | 2 | −4.83 | −8.85, −0.82 | 0.018 | 92.8 | < 0.001 | ||
| negative pressure type | Continuous nagative pressure | 12 | −10.09 | −11.11, −9.08 | < 0.001 | 56.2 | 0.009 | |
| Intermittent negative pressure | 9 | −10.08 | −12.93, −7.22 | < 0.001 | 94.0 | < 0.001 | ||
| unknown | 6 | −7.09 | −10.49, −3.69 | < 0.001 | 93.8 | < 0.001 | ||
| Mode of operation | NPWT before PRP | 19 | −9.39 | −10.65, −8.13 | < 0.001 | 87.4 | < 0.001 | |
| PRP before NPWT | 2 | −12.11 | −16.15, −8.06 | < 0.001 | 88.0 | 0.004 | ||
| Proceed simultaneously | 6 | −7.66 | −12.30, −3.03 | 0.001 | 94.1 | < 0.001 | ||
| Healing rate | Wound type | Mixed wound | 7 | 1.63 | 1.20, 2.21 | 0.002 | 84.7 | < 0.001 |
| Diabetic foot | 14 | 1.92 | 1.66, 2.23 | < 0.001 | 0.0 | 0.983 | ||
| Pressure injury | 2 | 1.45 | 0.94, 2.25 | 0.093 | 0.0 | 0.476 | ||
| negative pressure type | Continuous nagative pressure | 9 | 1.79 | 1.27, 2.52 | 0.001 | 55.9 | 0.020 | |
| Intermittent negative pressure | 5 | 1.58 | 1.22, 2.04 | < 0.001 | 55.9 | 0.013 | ||
| unknown | 9 | 1.91 | 1.46, 2.52 | < 0.001 | 63.0 | 0.006 | ||
| Mode of operation | NPWT before PRP | 18 | 1.97 | 1.63, 2.38 | < 0.001 | 50.5 | < 0.001 | |
| PRP before NPWT | 2 | 1.59 | 1.18, 2.15 | 0.002 | 10.9 | 0.289 | ||
| Proceed simultaneously | 3 | 1.23 | 1.07, 1.42 | 0.003 | 0.0 | 0.748 | ||
| Hospitalization costs | Wound type | Mixed wound | 1 | 6400.00 | 1187.82, 11,612.18 | 0.016 | — | — |
| Diabetic foot | 6 | 652.63 | −5808.77, 7114.03 | 0.843 | 100.0 | < 0.001 | ||
| Pressure injury | 0 | — | — | — | — | — | ||
| negative pressure type | Continuous nagative pressure | 3 | 2074.00 | −1.3e + 04, 16,913.13 | 0.784 | 100.0 | < 0.001 | |
| Intermittent negative pressure | 1 | −5300 | −6030.51, −4569.49 | < 0.001 | — | — | ||
| unknown | 3 | 2257.72 | −4525.87, 9041.31 | 0.514 | 99.9 | < 0.001 | ||
| Mode of operation | NPWT before PRP | 5 | −3121.01 | −1.0e + 04, 3831.87 | 0.379 | 100.0 | < 0.001 | |
| PRP before NPWT | 1 | 24,000.00 | 16,402.60, 31,597.40 | < 0.001 | — | — | ||
| Proceed simultaneously | 1 | 6400.00 | 1187.82, 11,612.18 | 0.016 | — | — | ||
| Pain score | Wound type | Mixed wound | — | |||||
| Diabetic foot | ||||||||
| Pressure injury | ||||||||
| negative pressure type | Continuous nagative pressure | 3 | −1.40 | −1.96, −0.83 | < 0.001 | 64.8 | 0.058 | |
| Intermittent negative pressure | 3 | −0.99 | −1.22, −0.75 | < 0.001 | 0.0 | 0.763 | ||
| unknown | 3 | −1.90 | −3.21, −0.60 | 0.004 | 97.1 | < 0.001 | ||
| Mode of operation | NPWT before PRP | 3 | −1.00 | −1.26, −0.73 | < 0.001 | 0.0 | 0.549 | |
| PRP before NPWT | 1 | −1.05 | −1.42, −0.68 | < 0.001 | — | — | ||
| Proceed simultaneously | 5 | −1.76 | −2.70, −0.83 | < 0.001 | 95.9 | < 0.001 | ||
| Length of Hospital stay | Wound type | Mixed wound | 8 | −10.00 | −13.55, −6.46 | < 0.001 | 96.8 | < 0.001 |
| Diabetic foot | 8 | −9.81 | −15.48, −4.14 | 0.001 | 99.2 | < 0.001 | ||
| Pressure injury | 0 | — | — | — | — | — | ||
| negative pressure type | Continuous nagative pressure | 6 | −10.81 | −15.08, −6.54 | < 0.001 | 93.9 | < 0.001 | |
| Intermittent negative pressure | 3 | −8.24 | −12.21, −4.26 | < 0.001 | 93.2 | < 0.001 | ||
| unknown | 7 | −9.82 | −16.09, −3.56 | < 0.001 | 99.5 | < 0.001 | ||
| Mode of operation | NPWT before PRP | 11 | −10.89 | −13.91, −7.87 | < 0.001 | 97.6 | < 0.001 | |
| PRP before NPWT | 2 | −6.22 | −17.37, 4.92 | 0.274 | 99.5 | < 0.001 | ||
| Proceed simultaneously | 3 | −8.37 | −11.01, −5.73 | < 0.001 | 0.0 | 0.598 | ||
| Number of dressing changing | Wound type | Mixed wound | 2 | −1.75 | −4.97, 1.48 | 0.288 | 99.5 | < 0.001 |
| Diabetic foot | 2 | −3.64 | −9.80, 2.53 | 0.248 | 98.6 | < 0.001 | ||
| Pressure injury | 0 | — | — | — | — | — | ||
| negative pressure type | Continuous nagative pressure | 1 | −3.40 | −3.84, −2.96 | < 0.001 | — | — | |
| Intermittent negative pressure | 1 | −0.11 | −0.24, 0.02 | 0.087 | — | — | ||
| unknown | 2 | −3.64 | −9.80, 2.53 | 0.248 | 98.6 | < 0.001 | ||
| Mode of operation | NPWT before PRP | 2 | −1.75 | −4.97, 1.48 | 0.288 | 99.5 | < 0.001 | |
| PRP before NPWT | 1 | −0.53 | −0.87, −0.19 | 0.003 | — | — | ||
| Proceed simultaneously | 1 | −6.82 | −8.24, −5.40 | < 0.001 | — | — | ||
| C‐reactive protein level, CRP | Wound type | Mixed wound | 6 | −0.81 | −0.94, −0.67 | < 0.001 | 3.0 | 0.397 |
| Diabetic foot | 0 | — | — | — | — | — | ||
| Pressure injury | 1 | −0.04 | −0.22, 0.14 | 0.655 | 88.8 | < 0.001 | ||
| negative pressure type | Continuous nagative pressure | 4 | −0.60 | −1.11, −0.09 | 0.021 | 93.4 | < 0.001 | |
| Intermittent negative pressure | 2 | −1.03 | −1.39, −0.68 | < 0.001 | 0.0 | 0.590 | ||
| unknown | 1 | −0.45 | −0.84, −0.06 | 0.025 | — | — | ||
| Mode of operation | NPWT before PRP | 3 | −0.44 | −1.00, 0.13 | 0.130 | 95.3 | < 0.001 | |
| PRP before NPWT | 1 | −0.91 | −1.48, −0.34 | 0.002 | — | — | ||
| Proceed simultaneously | 3 | −0.92 | −1.20, −0.64 | < 0.001 | 0.0 | 0.543 | ||
| Preparation time for secondary repair | Wound type | Mixed wound | — | |||||
| Diabetic foot | ||||||||
| Pressure injury | ||||||||
| negative pressure type | Continuous nagative pressure | 3 | −4.09 | −5.13, −3.04 | < 0.001 | 0.0 | 0.471 | |
| Intermittent negative pressure | 1 | −8.86 | −11.43, −6.29 | < 0.001 | — | — | ||
| unknown | 2 | −3.74 | −7.97, 0.50 | 0.084 | 91.4 | 0.001 | ||
| Mode of operation | NPWT before PRP | 3 | −3.70 | −5.88, −1.52 | 0.001 | 85.6 | 0.001 | |
| PRP before NPWT | 0 | — | — | — | — | — | ||
| Proceed simultaneously | 3 | −6.65 | −9.55, −3.75 | < 0.001 | 57.5 | 0.095 | ||
Abbreviations: 95% CI, confidence interval; I2, inconsistency; RR, Risk Ratio; WMD, weighted mean difference.
3.5. Sensitivity Analysis
The full results of sensitivity analyzes are showed in Appendix S6. After individually excluding each study, the effect size of the entire set of 13 measures remained statistically unchanged, thus demonstrating the robustness of the findings. The outcome of incidence of complications was found that a significant decrease in heterogeneity between studies after excluding Qian, 2021 (RR0.36, 95% CI [0.23, 0.56], p < 0.001; I2 = 7.1%, p = 0.366).
3.6. Publication Bias
The results of publication bias are presented in Figure 4 and Table 4. The funnel plot of the effective rate and healing rate exhibited asymmetry. Begg and Egger's test revealed significant publication bias (p < 0.001, p < 0.001; p = 0.139, p < 0.001). The position of the funnel plot for healing time and length of hospital stay displayed approximate symmetry, with no evidence of publication bias according to Begg and Egger's test (p = 0.868, p = 0.097; p = 0.685, p = 0.246).
Figure 4.
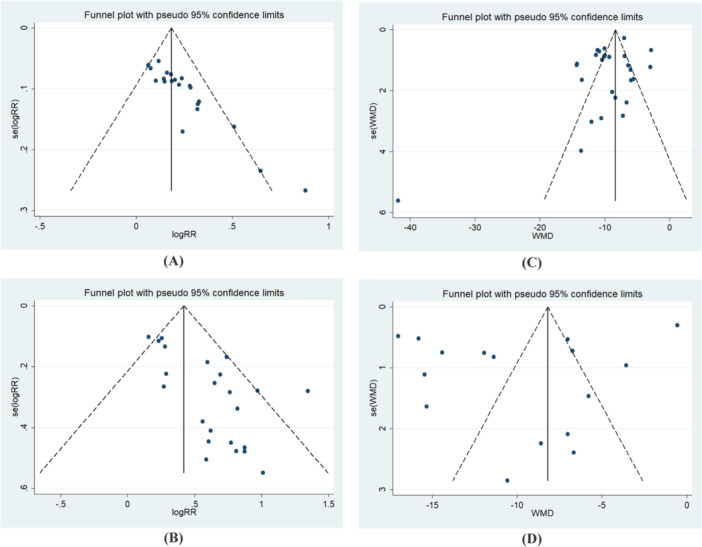
Funnel plot. (A) effective rate. (B) healing rate. (C) healing time. (D) Length of hospital stay.
Table 4.
Results of Begg and Egger's test.
| Outcomes | Begg's test | Egger's test | ||
|---|---|---|---|---|
| Z‐value | p‐value | t‐value | p‐value | |
| Effective rate | 4.34 | < 0.001 | 8.80 | < 0.001 |
| Healing rate | 1.48 | 0.139 | 4.61 | < 0.001 |
| Healing time | 0.17 | 0.868 | −1.72 | 0.097 |
| Length of hospital stay | 0.41 | 0.685 | −1.21 | 0.246 |
3.7. Certainty of Evidence
This study used GRADE to assess the quality of the certainty of evidence for each outcome. The quality of evidence was rated as moderate for 3 outcomes, low for 2, very low for 9, and high for none. A detailed summary of the certainty assessment for each outcome can be found in Table 5 and Appendix S7.
Table 5.
Summary of certainty of evidence assessment for each outcome using GRADE.
| Outcomes (Number of studies) | Grading of recommendations assessment, development, and evaluation | Number of participants | Effect 95%CI | QoE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Rob | Inc. | Ind. | Imp. | Pob | Int. | Con. | |||
| Effective rate (22) | ⊝ | ⊝ | ⊝ | ⊕ | ⊝ | 823 | 825 | RR 1.23 [1.17, 1.30] | Very low |
| Healing time (27) | ⊝ | ⊝ | ⊝ | ⊕ | ⊕ | 971 | 976 | WMD‐9.32 [−10.60, −8.03] | Very low |
| Healing rate (23) | ⊝ | ⊝ | ⊝ | ⊕ | ⊝ | 812 | 818 | RR1.76 [1.50, 2.07] | Very low |
| Length of Hospital stay (16) | ⊝ | ⊝ | ⊝ | ⊕ | ⊕ | 591 | 594 | WMD‐9.88 [−13.42, −6.34] | Very low |
| Number of dressing changing (4) | ⊝ | ⊝ | ⊝ | ⊕ | ⊕ | 160 | 162 | WMD‐2.56 [−4.28, −0.83] | Very low |
| Hospitalization costs (7) | ⊝ | ⊝ | ⊝ | ⊝ | ⊕ | 273 | 275 | WMD1423.56 [−4588.93, 7436.06] | Very low |
| Positive rate of bacterial (6) | ⊝ | ⊝ | ⊝ | ⊝ | ⊕ | 151 | 152 | RR0.25 [0.15, 0.40] | Very low |
| White blood cell level, WBC (5) | ⊝ | ⊕ | ⊕ | ⊕ | ⊕ | 133 | 134 | WMD‐1.71 [−2.00, −1.41] | Moderate |
| C‐reactive protein level, CRP (7) | ⊝ | ⊝ | ⊝ | ⊕ | ⊕ | 218 | 218 | WMD‐0.68 [−1.04, −0.33] | Very low |
| Erythrocyte sedimentation rate, ESR (3) | ⊝ | ⊕ | ⊕ | ⊕ | ⊕ | 75 | 75 | WMD‐6.09 [−8.05, −4.13] | Moderate |
| Pain score, 14 d (9) | ⊝ | ⊝ | ⊕ | ⊕ | ⊕ | 237 | 237 | WMD‐1.43 [−2.14, −0.72] | Low |
| Score of VSS (5) | ⊝ | ⊕ | ⊕ | ⊕ | ⊕ | 146 | 146 | WMD‐1.78 [−1.89, −1.66] | Moderate |
| Incidence of complications (7) | ⊝ | ⊝ | ⊝ | ⊕ | ⊕ | 317 | 317 | RR0.45 [0.30, 0.68] | Very low |
| Preparation time for secondary repair (6) | ⊝ | ⊝ | ⊕ | ⊕ | ⊕ | 173 | 173 | WMD‐4.95 [−7.03, −2.87] | Low |
Abbreviations: CI, confidence interval; Inc, inconsistency; Imp, imprecision; Ind, indirectness; PoB, publication of bias; Rob, risk of bias; QoE, quality of evidence; ⊝, represents degradation; ⊕, represents no degradation.
4. Discussion
The findings of this study indicate that the integration of NPWT with PRP significantly improves both the efficacy and healing rates of CRWs in comparison to the control group. This combination therapy also leads to a reduction in healing time, preparation time for secondary repair, and scar severity. NPWT, which employs negative pressure to facilitate wound debridement and drainage [60], has been demonstrated in animal studies to induce micro‐deformation shear stress and establish pressure gradients. These effects facilitate the regulation of gene expression involved in the development of the lymphatic network and enhance wound blood flow, exceeding four times the baseline levels [61, 62, 63]. Additionally, NPWT is known to stimulate cellular proliferation [64], augment the synthesis of growth factors and matrix metalloproteinases [65, 66, 67, 68], and support the maturation and stabilization of wound microvasculature by increasing angiopoietin‐2 expression levels as well as tyrosine kinase receptor 2 phosphorylation levels [69]. Despite the ongoing elucidation of the mechanisms involved, given variable findings, NPWT is associated with significant alterations in gene expression within the wound bed, influencing immune modulation and angiogenesis [70]. Meanwhile, PRP, as a concentrated source of platelets, provides a sustained milieu rich in growth factors crucial for CRW repair [71]. It activates platelet function to release a spectrum of growth factors and cytokines, and contains diverse components (alpha particles, delta particles containing serotonin, histamine, dopamine calcium adenosine, growth factors such as PDGF, EGF, TGF, VEGF, et al.) that synergistically govern the wound healing process [72, 73]. PRP is abundant in fibrinogen serotonin fibronectin factor V, VIII, and IV that form a fibrin matrix, which facilitates tissue infiltration, thereby potentially accelerating wound healing and minimizing scar formation [74].
Inflammation is a critical component of the wound healing process, and excessive inflammatory mediators can disrupt the delicate healing cascade and increase infection risks [75, 76]. Therefore, reducing bacterial load is vital for the effective management of CRWs. According to the study, combining NPWT with PRP effectively reduced local (positive rate of bacterial, rate of complications), systemic inflammatory responses (CRP, ESR, WBC) and patient pain more than control group. NPWT aids in eliminating niches for bacterial colonization and disrupts biofilm formation by continuously removing necrotic tissue through negative pressure suction. Moreover, it significantly reduces the translocation of bacteria, toxins, and inflammatory mediators into systemic circulation, thus preventing bacteremia and sepsis and enhancing patients’ overall health status to enable further therapeutic interventions [77]. Various studies have shown that NPWT can modulate oxidative stress and activate pathways such as the Rho‐Rho‐Kinase, ERK/MAPK, Cyclooxygenase, and ion acceptor pathways, leading to reduced local and systemic inflammation and promoting wound healing [78, 79, 80, 81]. PRP facilitates platelet activation, resulting in the release of peptides with intrinsic antimicrobial properties. These peptides effectively suppress the transcriptional activity of inflammatory mediators and C‐X‐C chemokine receptor 4, thus regulating inflammation and providing bactericidal effects [82]. Additionally, PRP modulates the expression levels of tissue inhibitor of metalloproteinases‐1 (TIMP‐1), matrix metalloproteinase‐9 (MMP‐9), and specific proteins in granulation tissue, while simultaneously reducing inflammatory cytokines such as interleukin‐1 beta (IL‐1β), interleukin‐8 (IL‐8), and tumor necrosis factor‐alpha (TNF‐α). By harnessing the synergistic anti‐inflammatory effects of NPWT and PRP, this combined therapeutic approach effectively manages wound inflammation and minimizes pain stimuli for enhanced patient outcomes.
Controversy remains over the optimal sequencing of NPWT and PRP for CRWs. Literature analysis highlights the essential pretreatment steps: glycemic control, lipid regulation, blood pressure management, and targeted anti‐infection therapy. Regular debridement using sharp, mechanical, or autolytic methods is crucial. Studies diverge on treatment order: some suggest starting with NPWT—incorporating foam trimming, transparent film, and connection to negative pressure devices (−450 to −125 mmHg) for 3–10 days—followed by PRP; others recommend administering 5–40 mL of PRP first, with a subsequent 1–3 days of negative pressure foam dressing before NPWT. A simultaneous application of both therapies has also been proposed. Our subgroup analysis indicates higher effective rate, increased CRP levels, and more frequent dressing changes with NPWT‐first, while PRP‐first resulted in prolonged hospital stays. Therefore, we advocate starting with NPWT to adequately prepare the wound bed before PRP application. The integrated analysis results demonstrated the superiority of both NPWT and PRP over the control group, thereby the available resources could be considered for the selection of treatment modalities. However, due to insufficient data, in‐depth analyzes of negative pressure parameters, PRP dosage, and the timing of combined treatment approaches have not been conducted, indicating a need for further research.
5. Limitations
This study has several limitations. Firstly, restricting inclusion to studies published in English and Chinese and focusing exclusively on research conducted in China limits the ability to generalize the findings regarding the efficacy and safety of NPWT combined with PRP for CRWs patients in other countries. This limitation arises due to the early adoption and significant application of these technologies within China, coupled with the prevalent issue of CRWs. Chinese researchers have concentrated considerable attention and resources on this area, facilitated by extensive academic collaborations. Conversely, research outside China remains limited, potentially influenced by factors such as timing of technology adoption, research priorities, resource distribution, and scholarly exchange. Additionally, there are concerns about the quality of the included studies, as many lack adequate descriptions of randomization methods and fail to implement allocation concealment or blinding. Furthermore, most studies address mixed wound types within the CRWs category, which obstructs subgroup analysis and may introduce result deviations due to wound heterogeneity. Moreover, insufficient reporting on aspects such as negative pressure suction type, method, material, and source may contribute to study heterogeneity. Although this study confirms the effectiveness and safety of NPWT combined with PRP in treating CRWs, it is recommended to conduct high‐quality multi‐center randomized controlled trials that can establish standardization of treatment protocols and optimize the utilization of NPWT and PRP therapies. These studies should encompass diverse patient populations, employ rigorous randomization and double‐blind methodologies, clearly define interventions and control groups while utilizing standardized outcome measures to yield more targeted and comparable clinical evidence. Moreover, current inconsistencies in treatment outcomes and the absence of standard treatment protocols necessitate development of standardized management strategies and an investigation into the mechanism of NPWT combined with PRP therapy, in line with CRWs pathogenesis.
6. Conclusion
CRWs remain a challenging issue in clinical treatment and nursing. NPWT induces local reactions through negative pressure, while PRP aids in reducing the inflammatory response of CRWs and promotes granulation and epithelial growth. This study discovered that combining NPWT with PRP significantly enhances the effectiveness and healing rate of CRWs, shortens healing time and secondary repair preparation time, reduces scarring, inflammation, bacterial culture positivity rate, pain intensity, length of hospital stays, number of dressing changes and incidence of complications compared to using NPWT alone. Moreover, there is no increase in hospitalization costs among patients. In the future, it is imperative to conduct more standardized, high‐quality, large‐scale, multicenter randomized controlled trials to further validate these findings. Additionally, comprehensive exploration of optimal parameter combinations and elucidation of pathogenesis and treatment mechanisms will provide substantial evidence for the development of standardized management strategies and research.
Author Contributions
Ran Hao: conceptualization, data curation, formal analysis, methodology, project administration, software, writing–original draft. Mao Luo: methodology, validation, visualization. Yanting Xiao: methodology, writing–review and editing. Jing Li: data curation, investigation. Xinyue Lv: data curation, formal analysis, investigation, validation. Yumei Peng: data curation, formal analysis, investigation, validation. Yuxuan Wu: methodology, resources, supervision. Yan Shen: methodology, resources, supervision. Wei Jiang: conceptualization, project administration, resources, supervision, writing–review and editing.
Ethics Statement
The manuscript guarantor Ran Hao affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Wei Jiang affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Acknowledgments
This work was funded by Sichuan Medical Association Wound Diseases (TaiGe) special research project (No. 2023TG18).
Data Availability Statement
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request. All relevant data and materials are included in the manuscript.
References
- 1. Wei Z. R. and Huang G. T., “Progress in the Treatment of Chronic Wound and Discussion on the Integrated Surgical Wound Treatment Mode,” Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chinese Journal of Burns 35, no. 11 (2019): 824–827, 10.3760/cma.j.issn.1009-2587.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 2. Yao Z. X., Fu X. B., and Cheng B., “New Concept of Chronic Wound Healing: Advances in the Research of Wound Management in Palliative Care,” Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chinese Journal of Burns 36, no. 8 (2020): 754–757, 10.3760/cma.j.cn501120-20190929-00388. [DOI] [PubMed] [Google Scholar]
- 3. Tan Q. and Xu Y., “Theories and Strategies of Chronic Wound Treatment,” Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chinese Journal of Burns 36, no. 9 (2020): 798–802, 10.3760/cma.j.cn501120-20200728-00361. [DOI] [PubMed] [Google Scholar]
- 4. Tan Q., “To Further Improve the Understanding of Traditional Treatment Methods for Chronic Wounds,” Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chinese Journal of Burns 37, no. 5 (2021): 410–412, 10.3760/cma.j.cn501120-20210413-00128. [DOI] [PubMed] [Google Scholar]
- 5. Liu X., Dou G., Li Z., et al., “Hybrid Biomaterial Initiates Refractory Wound Healing via Inducing Transiently Heightened Inflammatory Responses,” Advanced Science 9, no. 21 (2022): e2105650, 10.1002/advs.202105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones R. E., Foster D. S., and Longaker M. T., “Management of Chronic Wounds‐2018,” Journal of the American Medical Association (Chicago, IL) 320, no. 14 (2018): 1481–1482, 10.1001/jama.2018.12426. [DOI] [PubMed] [Google Scholar]
- 7. Anchalia M., Upadhyay S., and Dahiya M., “Negative Pressure Wound Therapy With Instillation and Dwell Time and Standard Negative Pressure Wound Therapy in Complex Wounds: Are They Complementary or Competitive?,” Wounds: A Compendium of Clinical Research and Practice 32, no. 12 (2020): 84. [PubMed] [Google Scholar]
- 8. Kim P. J., Attinger C. E., Constantine T., et al., “Negative Pressure Wound Therapy With Instillation: International Consensus Guidelines Update,” International Wound Journal 17, no. 1 (2020): 174–186, 10.1111/iwj.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadeghi‐Ataabadi M., Mostafavi‐Pour Z., Vojdani Z., Sani M., Latifi M., and Talaei‐Khozani T., “Fabrication and Characterization of Platelet‐Rich Plasma Scaffolds for Tissue Engineering Applications,” Materials Science and Engineering: C 71 (2017): 372–380, 10.1016/j.msec.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 10. Everts P. A., van Erp A., DeSimone A., Cohen D. S., and Gardner R. D., “Platelet Rich Plasma in Orthopedic Surgical Medicine,” Platelets 32, no. 2 (2021): 163–174, 10.1080/09537104.2020.1869717. [DOI] [PubMed] [Google Scholar]
- 11. Li F. L., Wu C. B., Sun H. J., and Zhou Q., “Comparison of Autologous Platelet‐Rich Plasma and Chitosan in the Treatment of Temporomandibular Joint Osteoarthritis: A Retrospective Cohort Study,” Journal of Oral and Maxillofacial Surgery 79, no. 2 (2021): 324–332, 10.1016/j.joms.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 12. Sahli E., Arslan U., Özmert E., and İdil A., “Evaluation of the Effect of Subtenon Autologous Platelet‐Rich Plasma Injections on Visual Functions in Patients With Retinitis Pigmentosa,” Regenerative Medicine 16, no. 2 (2021): 131–143, 10.2217/rme-2020-0075. [DOI] [PubMed] [Google Scholar]
- 13. de Miguel–Gómez L., López‐Martínez S., Campo H., et al., “Comparison of Different Sources of Platelet‐Rich Plasma as Treatment Option for Infertility‐Causing Endometrial Pathologies,” Fertility and Sterility 115, no. 2 (2021): 490–500, 10.1016/j.fertnstert.2020.07.053. [DOI] [PubMed] [Google Scholar]
- 14. Yin S., Yang X., Bi H., and Zhao Z., “Combined Use of Autologous Stromal Vascular Fraction Cells and Platelet‐Rich Plasma for Chronic Ulceration of the Diabetic Lower Limb Improves Wound Healing,” The International Journal of Lower Extremity Wounds 20, no. 2 (2021): 135–142, 10.1177/1534734620907978. [DOI] [PubMed] [Google Scholar]
- 15. Chen H., Xu T. J., Yu H., Zhu J. L., Liu Y., and Yang L. P., “Retracted: Effect of Platelet‐Rich Plasma Combined With Negative Pressure Wound Therapy in Treating Patients With Chronic Wounds: A Meta‐Analysis,” International Wound Journal 21, no. 4 (2024): e14758, 10.1111/iwj.14758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Page M. J., McKenzie J. E., Bossuyt P. M., et al., “The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews,” BMJ 372 (2021): n71, 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne J. A. C., Savović J., Page M. J., et al., “Rob 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials,” BMJ 366 (2019): l4898, 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt G. H., Oxman A. D., Kunz R., Vist G. E., Falck‐Ytter Y., and Schünemann H. J., “What is “Quality of Evidence” and Why is it Important to Clinicians?,” BMJ 336, no. 7651 (2008): 995–998, 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guyatt G. H., Oxman A. D., Vist G. E., et al., “Grade: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations,” BMJ 336, no. 7650 (2008): 924–926, 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt G. H., Oxman A. D., Vist G. E., et al., “Grade Guidelines: Ⅳ. Grading of Quality of Evidence—Limitations of Studies (Risk of Bias),” Chinese Journal of Evidence‐Based Medicine 11, no. 4 (2011): 456–463, 10.3969/j.issn.1672-2531.2011.04.018. [DOI] [Google Scholar]
- 21. Guyatt G. H., Oxman A. D., Montori V., et al., “Grade Guideline: ⅴ. Quality of Evidence Assessment ‐ Publication Bias,” Chinese Journal of Evidence‐Based Medicine 11, no. 12 (2011): 1430–1434, 10.3969/j.issn.1672-2531.2011.12.015. [DOI] [Google Scholar]
- 22. Guyatt G. H., Oxman A. D., Kunz R., et al., “GRADE Guideline: Ⅵ. Evaluation of Evidence Quality: Imprecision (Random Error),” Chinese Journal of Evidence‐Based Medicine 11, no. 12 (2011): 1435–1443, 10.3969/j.issn.1672-2531.2011.12.016. [DOI] [Google Scholar]
- 23. Guyatt G. H., Oxman A. D., Kunz R., et al., “Grade Guideline: Ⅶ. Assessment of Evidence Quality—Inconsistency,” Chinese Journal of Evidence‐Based Medicine 11, no. 12 (2011): 1444–1451, 10.3969/j.issn.1672-2531.2011.12.017. [DOI] [Google Scholar]
- 24. Guyatt G. H., Oxman A. D., Kunz R., et al., “GRADE Guideline: VIII. Quality Assessment of Evidence—Indirectness,” Chinese Journal of Evidence‐Based Medicine 11, no. 12 (2011): 1452–1458, 10.3969/j.issn.1672-2531.2011.12.018. [DOI] [Google Scholar]
- 25. Li C., Jinmei L., Yuping Z., et al., “Clinical Effect of Autologous Platelet‐Rich Plasma Combined With Negative Pressure on Treatment of Diabetic Foot Infection,” Chinese Journal of Nosocomiology 31, no. 7 (2021): 1029–1033, 10.11816/cn.ni.2021-202118. [DOI] [Google Scholar]
- 26. Ling C., “Clinical Study of Platelet‐Rich Plasma Combined With Vacuum Sealing Drainage in the Treatment of Chronic Refractory Wounds” (MA thesis, Youjiang Medical College for Nationalities, 2022).
- 27. Xinchan C., Xiaojing W., Jiancai L., et al., “Therapeutic Effect of Autologous Platelet‐Rich Gel Combined With Continuous Vacuum Sealing Drainage on Diabetic Foot Ulcer,” Nursing Practice and Research 15, no. 11 (2018): 42–44, 10.3969/j.issn.1672-9676.2018.11.017. [DOI] [Google Scholar]
- 28. Changzhu C. and Ruiqiang W., “Efficacy of Autologous Platelet‐Rich Gel Combined With Continuous Closed Negative Pressure Drainage in the Treatment of Diabetic Foot Ulcer,” China Reflexolocy 27, no. 22 (2018): 42–43, 10.19589/j.cnki.issn1004-6569.2018.22.042. [DOI] [Google Scholar]
- 29. Peng F., Ting M., and Zaidao Y., “Effects of Autologous Platelet‐Rich Gel Combined With Negative Pressure Drainage on Insulin Resistance Level and Wound Healing in Diabetic Foot Patients,” Hainan Medical Journal 30, no. 4 (2019): 448–451, 10.3969/j.issn.1003-6350.2019.04.012. [DOI] [Google Scholar]
- 30. Wenhua G., Jiangmin Z., Hongliang Z., et al., “Autologous Platelet‐Rich Gel Combined With Vacuum Sealing Drainage in the Treatment of Diabetic Foot Ulcers,” International Journal of Geriatrics 42, no. 4 (2021): 238–241, 10.3969/j.issn.1674-7593.2021.04.012. [DOI] [Google Scholar]
- 31. Feifei H., “Application of Self Platelet Rich Plasma Combined With Vacuum Sealing Drainage in the Treatment of Diabetic Foot Ulcer,” Health Required 2020, no. 6 (2020): 10. [Google Scholar]
- 32. Qunfang L., “To Observe the Effect of Negative Pressure Wound Therapy Combined With Autologous Platelet‐Rich Gel in the Treatment of Diabetic Ulcer,” Diabetes New World 23, no. 7 (2020): 28–30, 10.16658/j.cnki.1672-4062.2020.07.028. [DOI] [Google Scholar]
- 33. Rilun L., Xiaolian L., Hao J., et al., “To Observe the Effect of VSD Combined With Prp in the Treatment of Diabetic Foot Ulcer Wounds and Its Influence on Oxidative Stress Indexes of Patients,” Journal of Clinical Medical Literature 7, no. 87 (2020): 48–49. [Google Scholar]
- 34. Jinhu L., Zhaocheng S., Xinhe Z., et al., “To Observe the Clinical Effect of Autologous Platelet‐Rich Plasma Combined With Vacuum Sealing Drainage in the Treatment of Wounds With Bone Exposure in the Elderly,” Sichuan Medicine 39, no. 5 (2018): 586–589, 10.16252/j.cnki.issn1004-0501-2018.05.028. [DOI] [Google Scholar]
- 35. Zhongxing L., Jiangxin W., Jinfeng H., et al., “Effect of Platelet‐Rich Plasma in Repairing Chronic Refractory Wounds of Lower Limbs and the Influence on Appearance,” Chinese Journal of Aesthetic Medicine 31, no. 7 (2022): 51–55, 10.15909/j.cnki.cn61-1347/r.005172. [DOI] [Google Scholar]
- 36. Xionghua L., Xijiao Z., Weijun G., et al., “Clinical Observation of Vacuum Sealing Drainage Combined With Autologous Platelet Rich Gel in the Treatment of Type 2 Diabetic Foot Ulcers,” Electronic Journal of Foot and Ankie Surgery 8, no. 2 (2021): 45–47, 51. 10.3969/j.issn.2095-7793.2021.02.010. [DOI] [Google Scholar]
- 37. Chijiao M., “The Effect of Vacuum Sealing Drainage Combined With Platelet‐Rich Plasma in the Treatment of Refractory Wounds,” Shanxi Medical Journal 47, no. 18 (2018): 2180–2182, 10.3969/j.issn.0253-9926.2018.18.022. [DOI] [Google Scholar]
- 38. Hongwei M., “The Effect of Vacuum Sealing Drainage (VSD) Combined With Autologous Platelet‐Rich Plasma (Prp) in the Treatment of Diabetic Foot Ulcers,” Oriental Medicated Diet 10, no. 20 (2019): 62–63. [Google Scholar]
- 39. Jinpeng M., “Clinical Effect of VSD Combined With Platelet‐Rich Plasma in the Treatment of Refractory Wounds,” China Practical Medicine 13, no. 5 (2018): 117–118, 10.14163/j.cnki.11-5547/r.2018.05.069. [DOI] [Google Scholar]
- 40. Maisvuti M. M. T., Zhao C., Zhizhong W., et al., “Application Value of Autologous Platelet‐Rich Plasma Combined With Vacuum Sealing Drainage in the Treatment of Diabetic Foot Ulcers,” Journal of Medical Aesthetics and Cosmetology, no. 23 (2021): 72–73. [Google Scholar]
- 41. Rao P., Tao L., Lisha L., et al., “Clinical Effect of Autologous Platelet‐Rich Plasma Combined With Negative Pressure on Treatment of Diabetic Foot Infection,” Practical Journal of Clinical Medicine 17, no. 6 (2020): 113–116, 10.3969/j.issn.1672-6170.2020.06.033. [DOI] [Google Scholar]
- 42. Guoguang Q., “The Therapeutic Effect of Platelet‐Rich Plasma Combined With Negative‐Pressure Wound Therapy on Chronic Refractory Wounds,” Journal of Medical Aesthetics and Cosmetology 30, no. 3 (2021): 72–73. [Google Scholar]
- 43. Fuzeng S., Tianjian Z., Xiaolong L., et al., “Application of Vacuum Sealing Drainage Combined With Platelet‐Rich Plasma Technology in the Treatment of Patients With Diabetic Foot Disease,” Journal of Chinese Physician 23, no. 5 (2021): 776–778, 10.3760/cma.j.cn431274-20200508-00581. [DOI] [Google Scholar]
- 44. Quan S. and Hui L., “Application of Autologous Platelet‐Rich Gel Combined With Negative Pressure Assisted Closure Technique in the Treatment of Pressure Ulcers,” The Journal of Medical Theory and Practice 34, no. 2 (2021): 261–263, 10.19381/j.issn.1001-7585.2021.02.039. [DOI] [Google Scholar]
- 45. Guoyang W. and Quan S., “Effect of Autologous Platelet‐Rich Gel Combined With Negative Pressure Assisted Closure Technique in the Treatment of Diabetic Foot Ulcer,” Chinese Journal of Clinical Rational Drug Use 14, no. 23 (2021): 165–167, 10.15887/j.cnki.13-1389/r.2021.23.069. [DOI] [Google Scholar]
- 46. Zhenqiang W. and Rongyao Y., “Analysis of the Efficacy of Autologous Platelet‐Rich Plasma Gel in the Treatment of Refractory Wounds of Extremities,” Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 31, no. 1 (2021): 124–125, 10.16458/j.cnki.1007-0893.2021.01.059. [DOI] [Google Scholar]
- 47. Qingjian X., Zhou H., and Jin L., “Investigation of the Therapeutic Effect of Platelet‐Rich Plasma Combined With Negative‐Pressure Wound Therapy on Refractory Wounds,” Heilongjiang Medicine Journal no. 33 (2020): 669–670, 10.14035/j.cnki.hljyy.2020.03.089. [DOI] [Google Scholar]
- 48. Juan X., Hao D., Dongsheng C., et al., “Clinical Efficacy of Closed Drainage Combined With Autologous Platelet‐Rich Gel in the Treatment of Diabetic Foot,” Acta Universitatis Medicinalis Anhui 54, no. 3 (2019): 478–481, 10.19405/j.cnki.issn1000-1492.2019.03.029. [DOI] [Google Scholar]
- 49. Fei X., “Clinical Study of Platelet‐Rich Plasma Combined With Vacuum‐Assisted Closure Technique in the Treatment of Refractory Wounds,” (MA thesis, Nanchang University, 2020).
- 50. Biao Y., Shan W., Yan Z., et al., “Negative Pressure Wound Therapy Combined With Platelet‐Rich Plasma in the Treatment of Chronic Refractory Wounds: To Accelerate Wound Re‐Epithelialization and Healing Rate,” Journal of Clinical Rehabilitative Tissue Engineering Research 23, no. 26 (2019): 4181–4186, 10.3969/j.issn.2095-4344.1357. [DOI] [Google Scholar]
- 51. Guanlong Y., Liying W., Fanchao S., et al., “Clinical Study of Vacuum Sealing Drainage Combined With Autologous Platelet‐Rich Plasma in the Repair of Diabetic Cutaneous Chronic Refractory Wounds,” Youjiang Medical Journal 50, no. 3 (2022): 210–213, 10.3969/j.issn.1003-1383.2022.03.010. [DOI] [Google Scholar]
- 52. Ronghua Y., Zepeng L., Qi X., et al., “Effects of Negative Pressure Wound Therapy Combined With Platelet‐Rich Plasma on Pain Degree and Inflammatory Index in Patients With Chronic Refractory Wounds,” Heilongjiang Medicine Journal 33, no. 3 (2020): 523–526, 10.14035/j.cnki.hljyy.2020.03.015. [DOI] [Google Scholar]
- 53. Jixiang Y., Li Z., and Yan L., “Efficacy of Closed Negative Pressure Suction and Autologous Platelet‐Rich Gel in the Treatment of Diabetic Foot,” Journal of Practical Diabetology 11, no. 4 (2015): 27–29. [Google Scholar]
- 54. Xuecheng Z., Fanjun M., Dachuan Z., et al., “Vacuum Sealing Drainage Combined With Platelet‐Rich Plasma Implant in the Treatment of Diabetes Foot Ulcer,” Clinical Journal of Medical Officer 45, no. 12 (2017): 1261–1263, 10.16680/j.1671-3826.2017.12.15. [DOI] [Google Scholar]
- 55. Yaping Z., “Clinical Observation and Nursing of PRP Combined With VSD to Promote the Growth of Wound Granulation Tissue,” Electronic Journal of Practical Clinical Nursing Science 4, no. 51 (2019): 154. [Google Scholar]
- 56. Lihua Z., Qinghua C., Zongcun C., et al., “Therapeutic Effect of Autologous Platelet‐Rich Gel Combined With Vacuum Sealing Drainage in the Treatment of Foot Ulcers of Patients With Diabetes,” Chinese Journal of the Frontiers of Medical Science 9, no. 6 (2017): 131–134, 10.12037/YXQY.2017.06-30. [DOI] [Google Scholar]
- 57. Siwen Z., Li Z., Bang H., et al., “Platelet‐Rich Plasma Combined With Vacuum‐Sealing Drainage in Treating Chronic Refractory Wounds,” Chinese Journal of General Practice 19, no. 2 (2021): 205–208, 10.16766/j.cnki.issn.1674-4152.001768. [DOI] [Google Scholar]
- 58. Liu Q., Zhang N., Li Z., and He H., “Efficacy of Autologous Platelet‐Rich Plasma Gel in the Treatment of Refractory Pressure Injuries and Its Effect on Wound Healing Time and Patient Quality of Life,” Clinics 76 (2021): e2355, 10.6061/clinics/2021/e2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xue X., Bian Y., Yang M., et al., “Evaluation of Injectable Platelet‐Rich Fibrin Produced by a Simple Twice‐Centrifugation Method Combined With Vacuum Sealing Drainage Technology in the Treatment of Chronic Refractory Wounds,” Frontiers in Bioengineering and Biotechnology 10 (2022): 979834, 10.3389/fbioe.2022.979834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gethin G., Cowman S., and Kolbach D. N., “Debridement for Venous Leg Ulcers,” Cochrane Database of Systematic Reviews 2019, no. 9 (2015): CD008599, 10.1002/14651858.CD008599.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morykwas M. J., Argenta L. C., Shelton‐Brown E. I., and McGuirt W., “Vacuum‐Assisted Closure: A New Method for Wound Control and Treatment: Animal Studies and Basic Foundation,” Annals of Plastic Surgery 38, no. 6 (1997): 553–562, 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 62. Yuan Y., Niu Y., Xiao W., Qi B., Hu X., and Yu A., “The Effect and Mechanism of Negative Pressure Wound Therapy on Lymphatic Leakage in Rabbits,” Journal of Surgical Research 235 (2019): 329–339, 10.1016/j.jss.2018.09.065. [DOI] [PubMed] [Google Scholar]
- 63. Randolph G. J., Ivanov S., Zinselmeyer B. H., and Scallan J. P., “The Lymphatic System: Integral Roles in Immunity,” Annual Review of Immunology 35 (2017): 31–52, 10.1146/annurev-immunol-041015-055354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hsiao H. Y., Hsieh W. C., Chang F. C. S., et al., “The Effect of Negative Pressure on Wound Healing and Regeneration in Closed Incisions Under High Tension: Evidence From Animal Studies and Clinical Experience,” Journal of Clinical Medicine 12, no. 1 (2022): 106, 10.3390/jcm12010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dasu M. R. K., Barrow R. E., Spies M., and Herndon D. N., “Matrix Metalloproteinase Expression in Cytokine Stimulated Human Dermal Fibroblasts,” Burns 29, no. 6 (2003): 527–531, 10.1016/s0305-4179(03)00154-2. [DOI] [PubMed] [Google Scholar]
- 66. Murphy M. A., Joyce W. P., Condron C., and Bouchier‐Hayes D., “A Reduction in Serum Cytokine Levels Parallels Healing of Venous Ulcers in Patients Undergoing Compression Therapy,” European Journal of Vascular and Endovascular Surgery 23, no. 4 (2002): 349–352, 10.1053/ejvs.2002.1597. [DOI] [PubMed] [Google Scholar]
- 67. Vaalamo M., Leivo T., and Saarialho‐Kere U., “Differential Expression of Tissue Inhibitors of Metalloproteinases (TIMP‐1, −2, −3, and −4) in Normal and Aberrant Wound Healing,” Human Pathology 30, no. 7 (1999): 795–802, 10.1016/s0046-8177(99)90140-5. [DOI] [PubMed] [Google Scholar]
- 68. Trengove N. J., Bielefeldt‐Ohmann H., and Stacey M. C., “Mitogenic Activity and Cytokine Levels in Non‐Healing and Healing Chronic Leg Ulcers,” Wound Repair and Regeneration 8, no. 1 (2000): 13–25, 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 69. Ma Z., Shou K., Li Z., Jian C., Qi B., and Yu A., “Negative Pressure Wound Therapy Promotes Vessel Destabilization and Maturation at Various Stages of Wound Healing and Thus Influences Wound Prognosis,” Experimental and Therapeutic Medicine 11, no. 4 (2016): 1307–1317, 10.3892/etm.2016.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ravindhran B., Schafer N., Howitt A., Carradice D., Smith G., and Chetter I., “Molecular Mechanisms of Action of Negative Pressure Wound Therapy: A Systematic Review,” Expert Reviews in Molecular Medicine 25 (2023): e29, 10.1017/erm.2023.24. [DOI] [PubMed] [Google Scholar]
- 71. Gentile P., Calabrese C., De Angelis B., et al., “Impact of the Different Preparation Methods to Obtain Autologous Non‐Activated Platelet‐Rich Plasma (A‐PRP) and Activated Platelet‐Rich Plasma (AA‐PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation,” International Journal of Molecular Sciences 21, no. 2 (2020): 431, 10.3390/ijms21020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nolan G. S., Smith O. J., Jell G., and Mosahebi A., “Fat Grafting and Platelet‐Rich Plasma in Wound Healing: A Review of Histology From Animal Studies,” Adipocyte 10, no. 1 (2021): 80–90, 10.1080/21623945.2021.1876374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lacci K. M. and Dardik A., “Platelet‐Rich Plasma: Support for Its Use in Wound Healing,” The Yale Journal of Biology and Medicine 83, no. 1 (2010): 1–9. [PMC free article] [PubMed] [Google Scholar]
- 74. Li Xiaohui and Huang Xiangyan, “Application of Autologous Platelet‐Rich Plasma in the Treatment of Chronic Refractory Wounds,” Shandong Medicine 63, no. 04 (2023): 107–110. [Google Scholar]
- 75. Alves R. and Grimalt R., “A Review of Platelet‐Rich Plasma: History, Biology, Mechanism of Action, and Classification,” Skin Appendage Disorders 4, no. 1 (2018): 18–24, 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gentile P. and Garcovich S., “Systematic Review: Adipose‐Derived Mesenchymal Stem Cells, Platelet‐Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects,” International Journal of Molecular Sciences 22, no. 4 (2021): 1538, 10.3390/ijms22041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hu F. X., Hu X. X., Yang X. L., et al., “Treatment of Large Avulsion Injury in Perianal, Sacral, and Perineal Regions by Island Flaps or Skin Graft Combined With Vacuum Assisted Closure,” BMC Surgery 19, no. 1 (2019): 65, 10.1186/s12893-019-0529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Qiu X., Wu Y., Zhang D., Zhang H., Yu A., and Li Z., “Roles of Oxidative Stress and Raftlin in Wound Healing Under Negative‐Pressure Wound Therapy,” Clinical, Cosmetic and Investigational Dermatology 14 (2021): 1745–1753, 10.2147/CCID.S334248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. İlhan E., Buyukafsar K., Tiftik R. N., Secilmis M. A., and Alzayed Z., “Effects of the Rho/Rho‐Kinase Pathway on Perfusion Pressure in the Isolated‐Perfused Rat Hind Limb Vascular Bed,” Balkan Medical Journal 38, no. 5 (2021): 304–309, 10.5152/balkanmedj.2021.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. An C., Huang Y., Li M., et al., “Vesicular Formation Regulated by ERK/MAPK Pathway Mediates Human Erythroblast Enucleation,” Blood Advances 5, no. 22 (2021): 4648–4661, 10.1182/bloodadvances.2021004859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Keringer P., Furedi N., Gaszner B., et al., “The Hyperthermic Effect of Central Cholecystokinin Is Mediated by the cyclooxygenase‐2 Pathway,” American Journal of Physiology‐Endocrinology and Metabolism 322, no. 1 (2022): E10–E23, 10.1152/ajpendo.00223.2021. [DOI] [PubMed] [Google Scholar]
- 82. Pires B. M. F. B., Baptista de Oliveira B. G. R., Bokehi L. C., et al., “Clinical and Microbiological Outcomes Associated With Use of Platelet‐Rich Plasma in Chronic Venous Leg Uclers: A Randomized Controlled Trial,” Journal of Wound, Ostomy & Continence Nursing 48, no. 4 (2021): 292–299, 10.1097/WON.0000000000000774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request. All relevant data and materials are included in the manuscript.


