Abstract
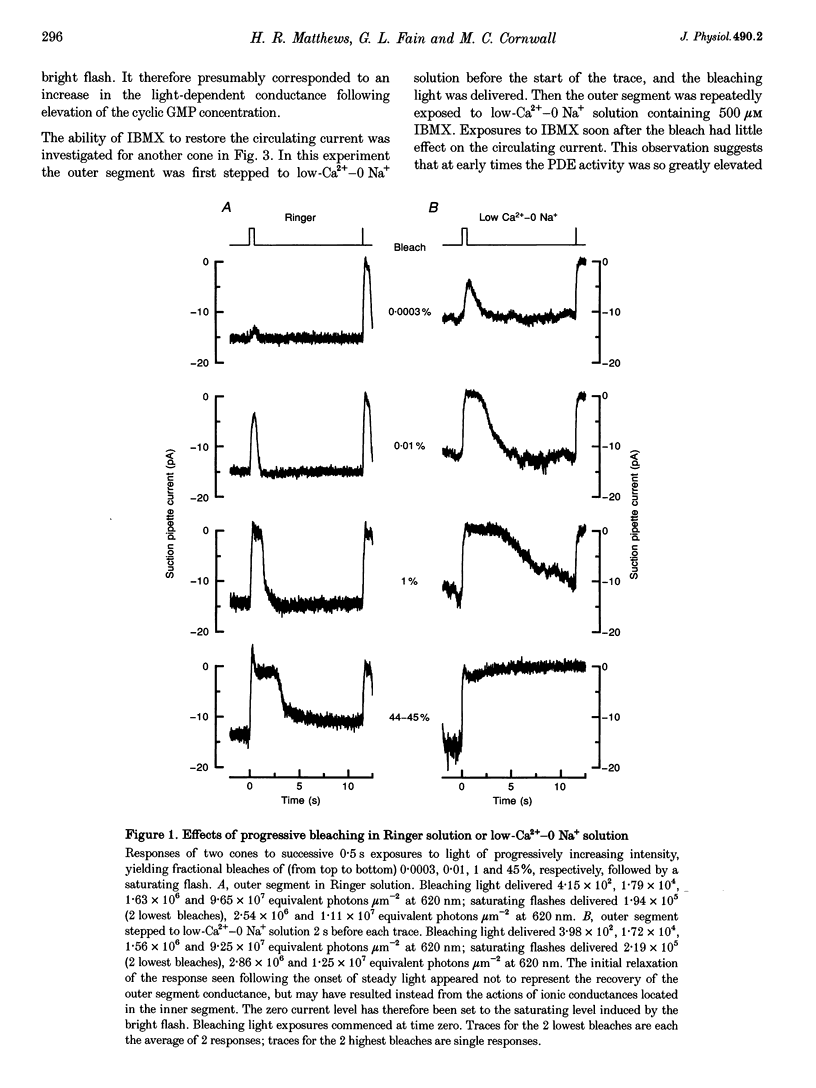
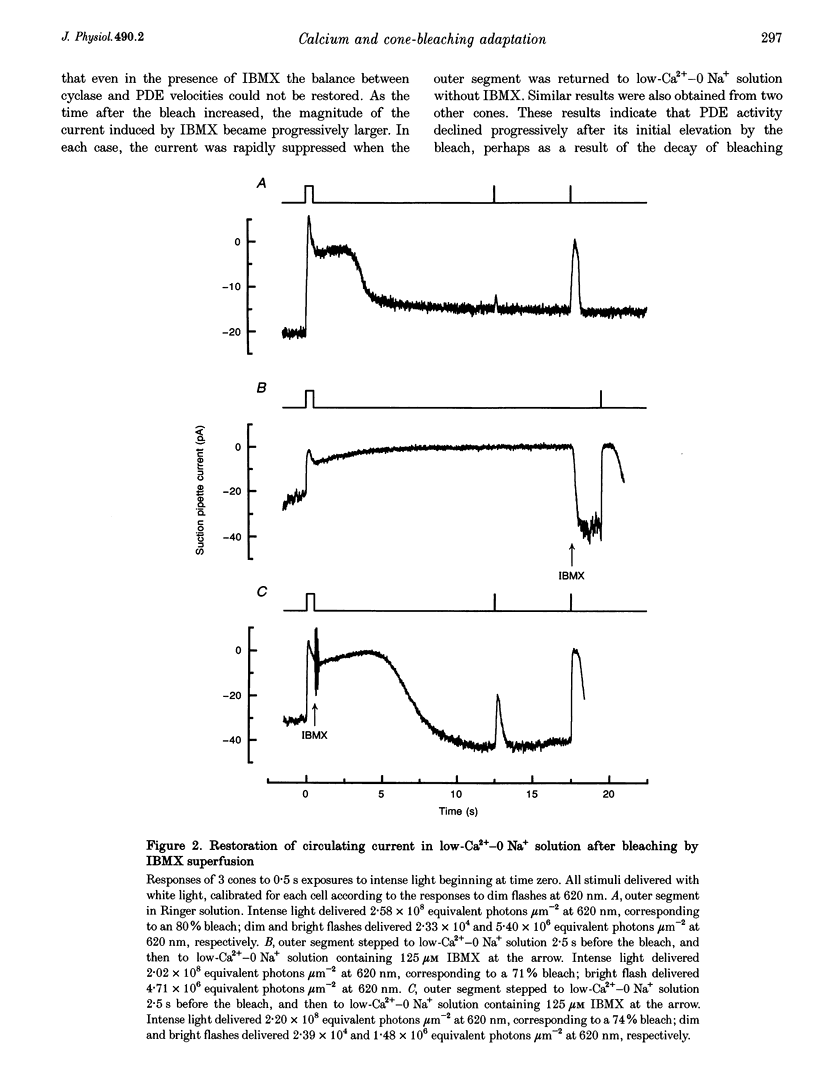
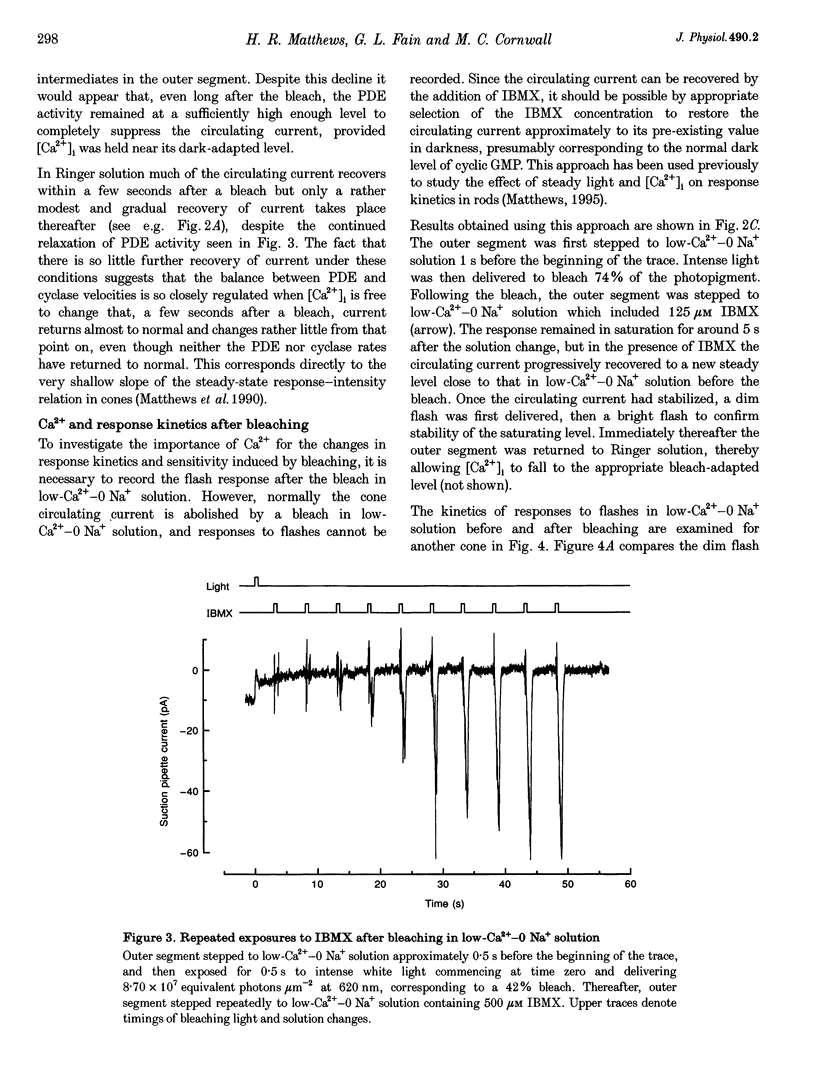
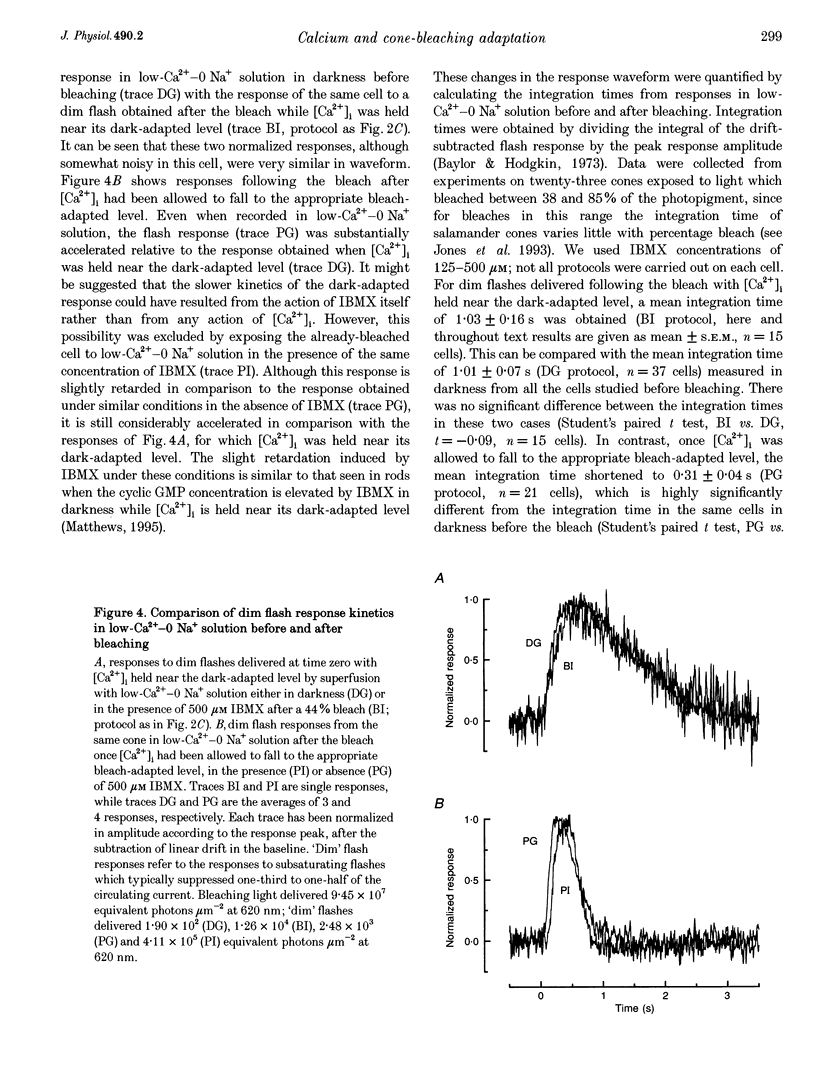
1. In order to study the possible involvement of Ca2+ in the bleaching adaptation of cones isolated from the retina of the salamander Ambystoma tigrinum, changes in cytoplasmic calcium concentration ([Ca2+]i were opposed by exposing the outer segment to a low-Ca(2+)-O Na+ solution designed to minimize Ca2+ fluxes across the outer segment membrane. 2. When a cone was exposed in normal Ringer solution to bright light bleaching a significant fraction of the photopigment, the circulating current was initially suppressed completely and then recovered to a maintained value less than the value in darkness before the bleach. When the outer segment of the cone was stepped to low-Ca(2+)-O Na+ solution before the bleach was delivered, the circulating current recovered more slowly or (for large bleaches) remained completely suppressed for the duration of the solution exposure. 3. If, during the period for which the current was suppressed in low-Ca(2+)-O Na+ solution, the cone outer segment was exposed to the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX), the circulating current was restored. The dim flash response recorded under these conditions exhibited kinetics and integration times similar to those recorded in low-Ca(2+)-O Na+ solution in darkness before the bleach. If, instead, the outer segment was returned to Ringer solution after the bleach, thereby allowing [Ca2+]i to fall from its dark-adapted level to the appropriate bleach-adapted level, the kinetics of the response in low-Ca(2+)-O Na+ solution were greatly accelerated, and the integration time considerably reduced. This was true regardless of whether or not the low-Ca(2+)-O Na+ solution included IBMX. 4. The role of Ca2+ in bleaching adaptation appeared to resemble its role in background adaptation, since in both cases exposure to low-Ca(2+)-O Na+ solution suppressed the acceleration of response kinetics. Responses recorded from cones in low-Ca(2+)-O Na+ solution were nearly identical in waveform and sensitivity during background light or after bleaches, provided that IBMX was used to restore sufficient photocurrent so that responses to flashes could be recorded, and sensitivity was corrected for loss in quantum catch. 5. These results indicate that the fall in [Ca2+]i in cones after a bleach is necessary both for the acceleration of the flash response and the adaptational decrease in sensitivity, as is the case for adaptation by background light.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Werblin F. S., Wilson M. The properties of single cones isolated from the tiger salamander retina. J Physiol. 1982 Jul;328:259–283. doi: 10.1113/jphysiol.1982.sp014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs W. H. Light and dark active phosphodiesterase regulation in salamander rods. J Gen Physiol. 1991 Sep;98(3):575–614. doi: 10.1085/jgp.98.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Fain G. L. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994 Oct 15;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Fein A., MacNichol E. F., Jr Cellular mechanisms that underlie bleaching and background adaptation. J Gen Physiol. 1990 Aug;96(2):345–372. doi: 10.1085/jgp.96.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Matthews H. R., Crouch R. K., Fain G. L. Bleached pigment activates transduction in salamander cones. J Gen Physiol. 1995 Sep;106(3):543–557. doi: 10.1085/jgp.106.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Ripps H., Chappell R. L., Jones G. J. Membrane current responses of skate photoreceptors. J Gen Physiol. 1989 Oct;94(4):633–647. doi: 10.1085/jgp.94.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lamb T. D., Matthews H. R., Murphy R. L. Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J Physiol. 1989 Sep;416:215–243. doi: 10.1113/jphysiol.1989.sp017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. Control of light-sensitive current in salamander rods. J Physiol. 1988 Sep;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Jones G. J., Fein A., MacNichol E. F., Jr, Cornwall M. C. Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. J Gen Physiol. 1993 Sep;102(3):483–502. doi: 10.1085/jgp.102.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R. External and internal actions in the response of salamander retinal rods to altered external calcium concentration. J Physiol. 1988 Sep;403:473–494. doi: 10.1113/jphysiol.1988.sp017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J Physiol. 1995 Apr 15;484(Pt 2):267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Fain G. L., Murphy R. L., Lamb T. D. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol. 1990 Jan;420:447–469. doi: 10.1113/jphysiol.1990.sp017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- McCarthy S. T., Younger J. P., Owen W. G. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of Fura-2. Biophys J. 1994 Nov;67(5):2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol. 1989 Feb;409:525–548. doi: 10.1113/jphysiol.1989.sp017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991 Feb;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]


