Abstract
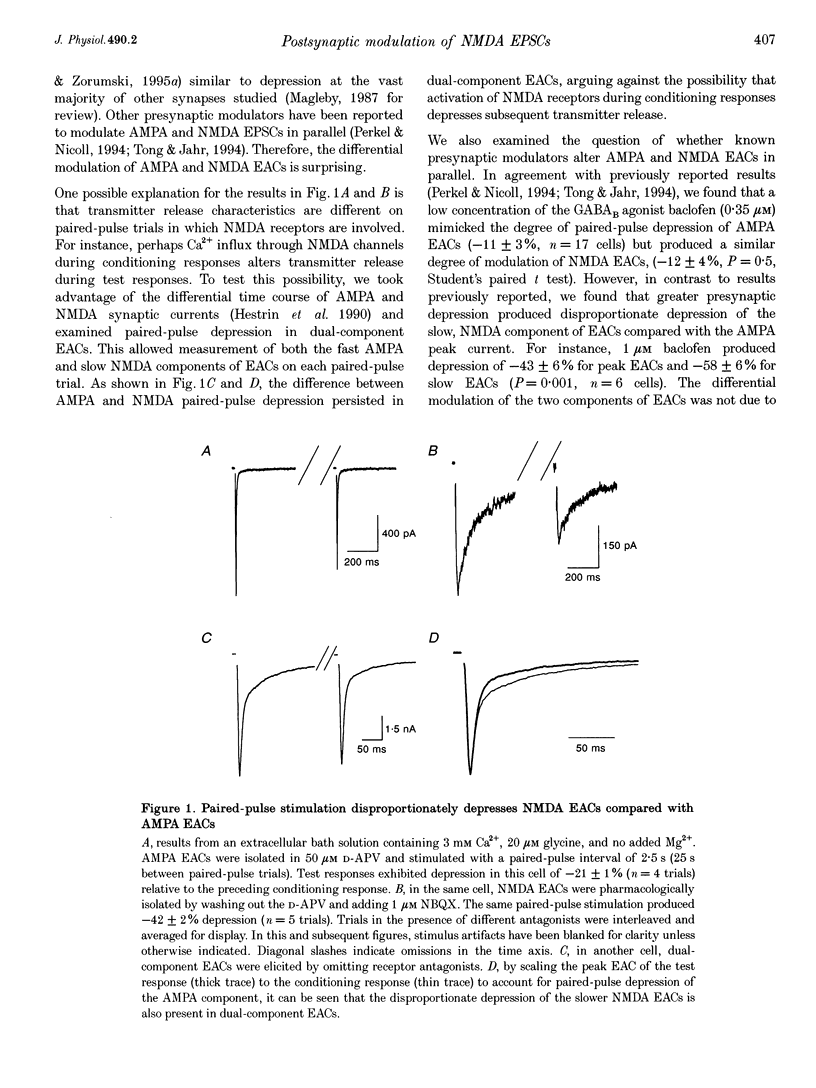
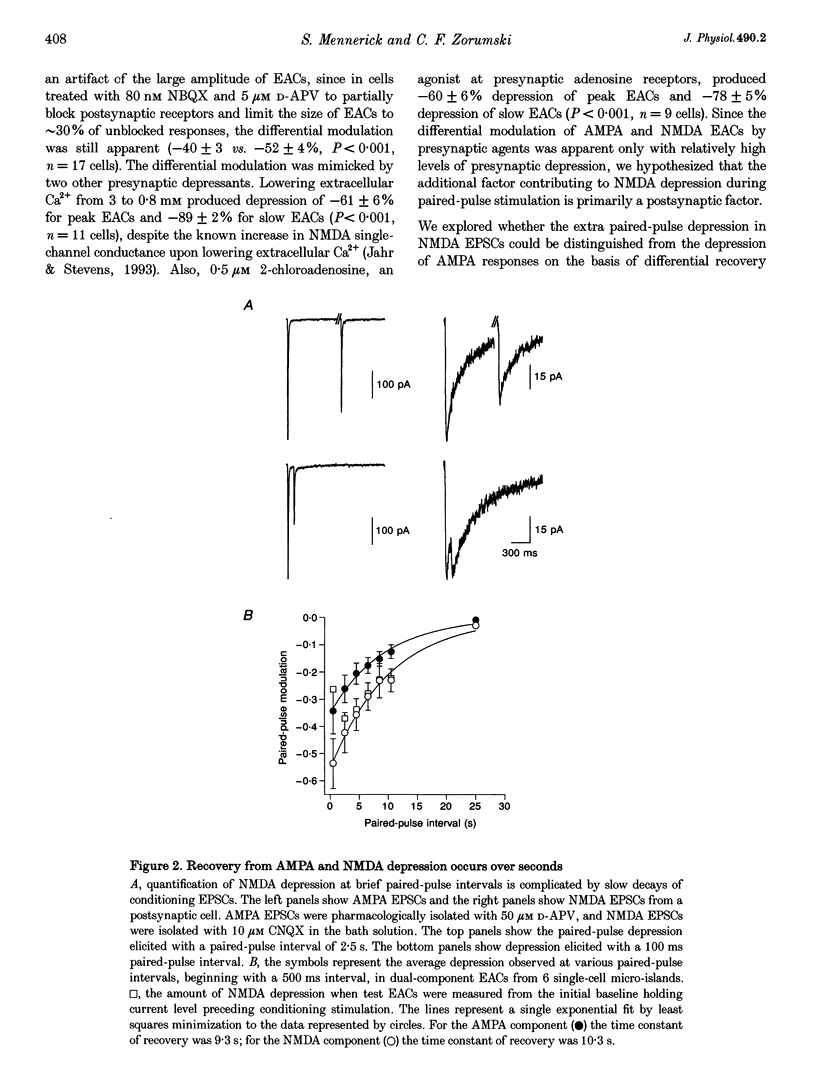
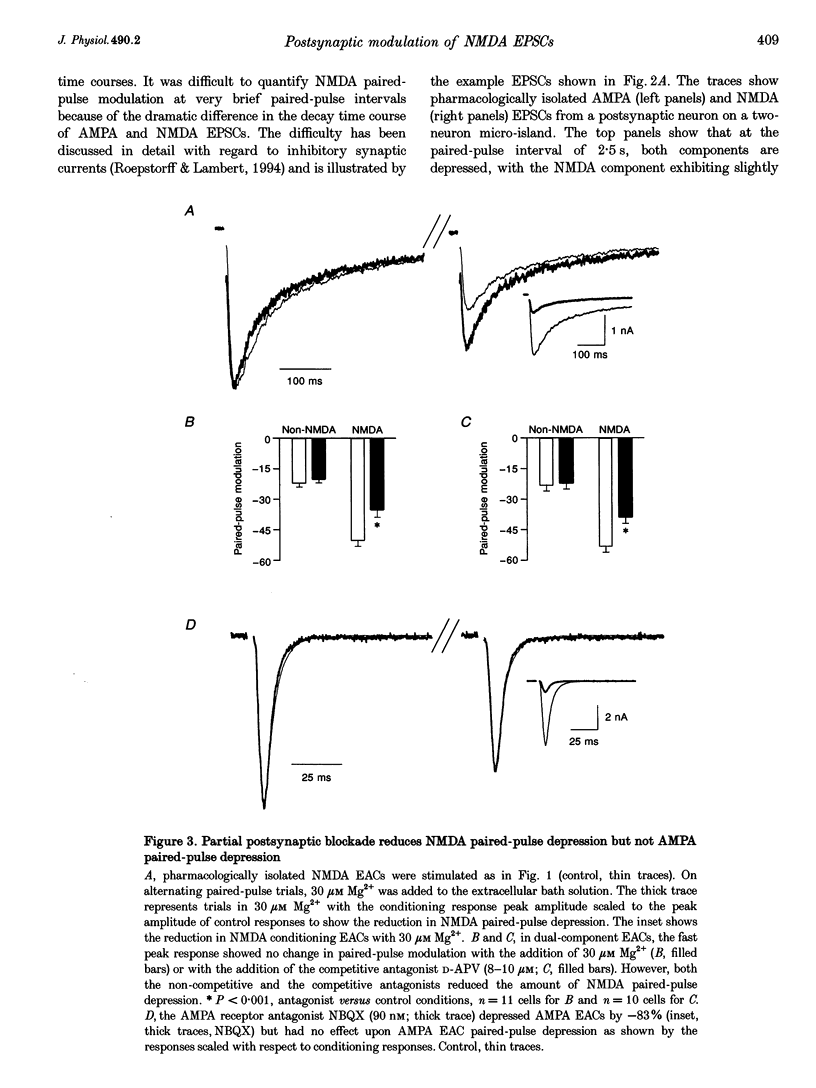
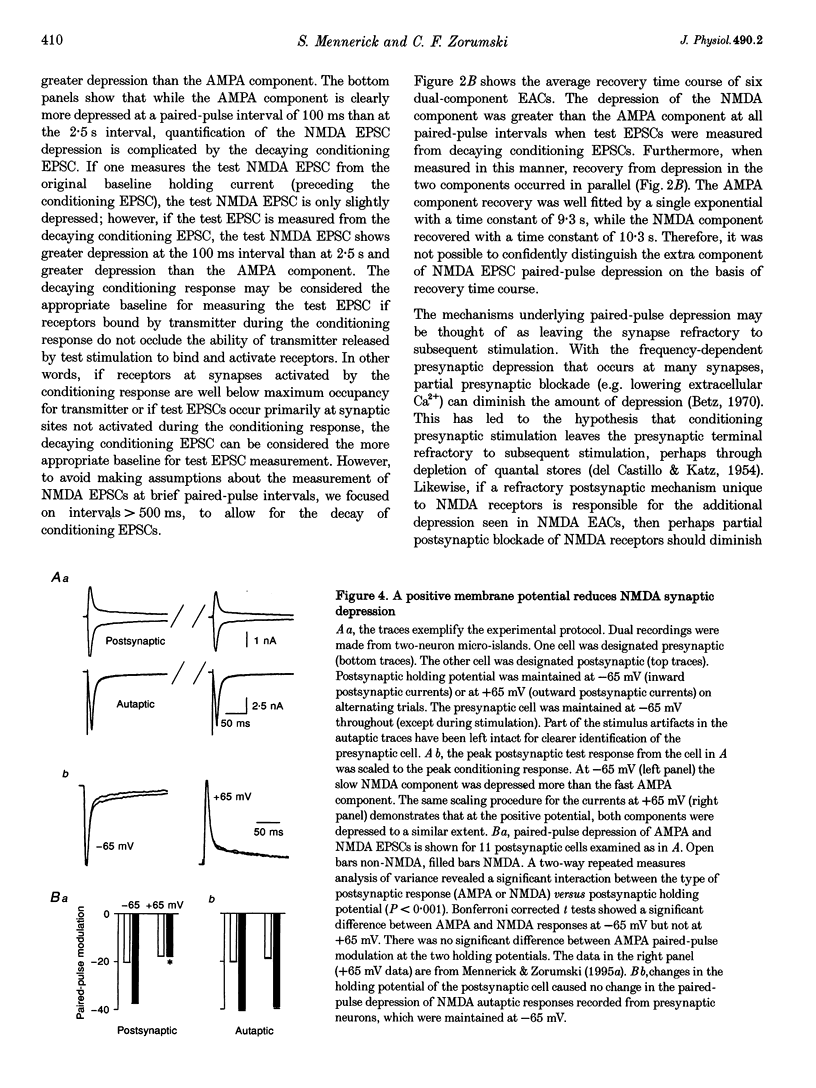
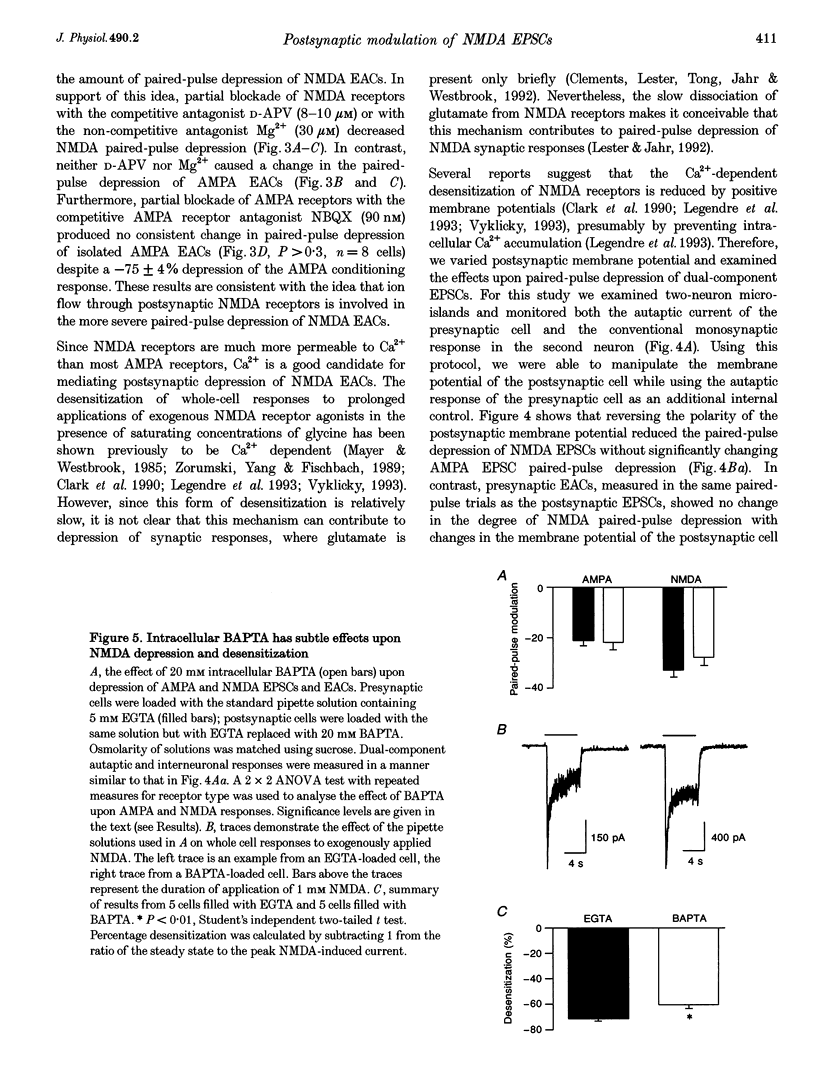
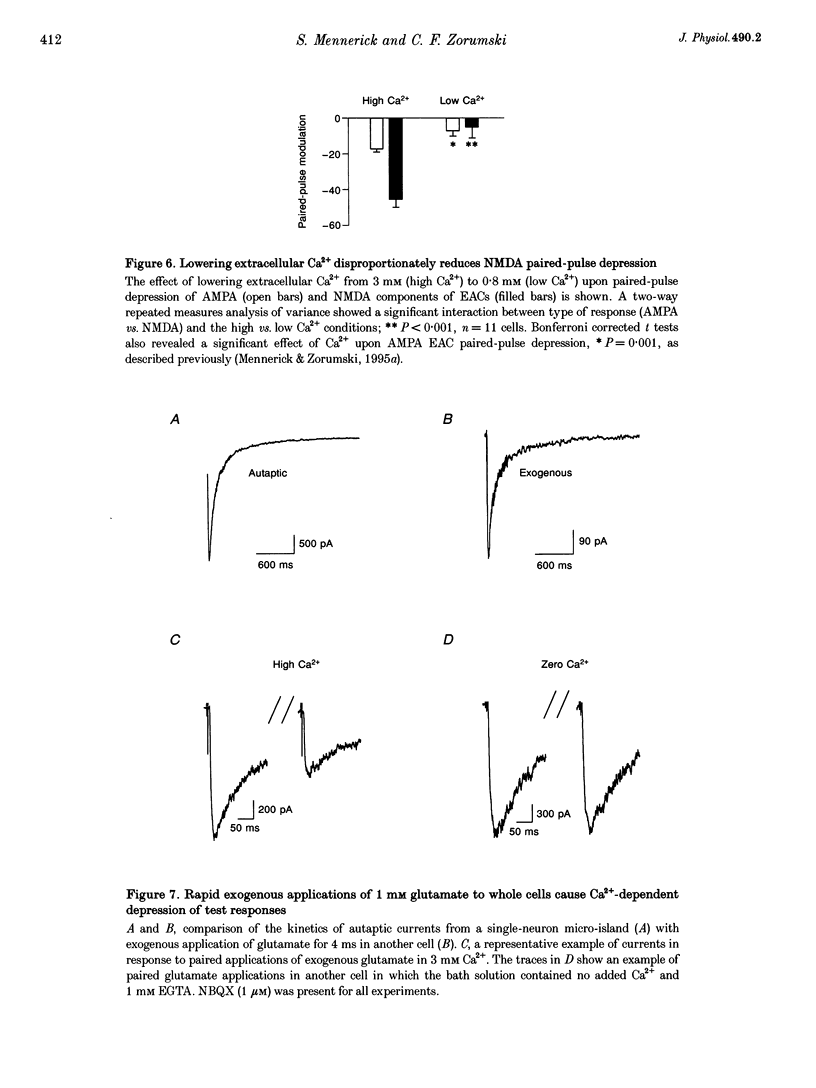
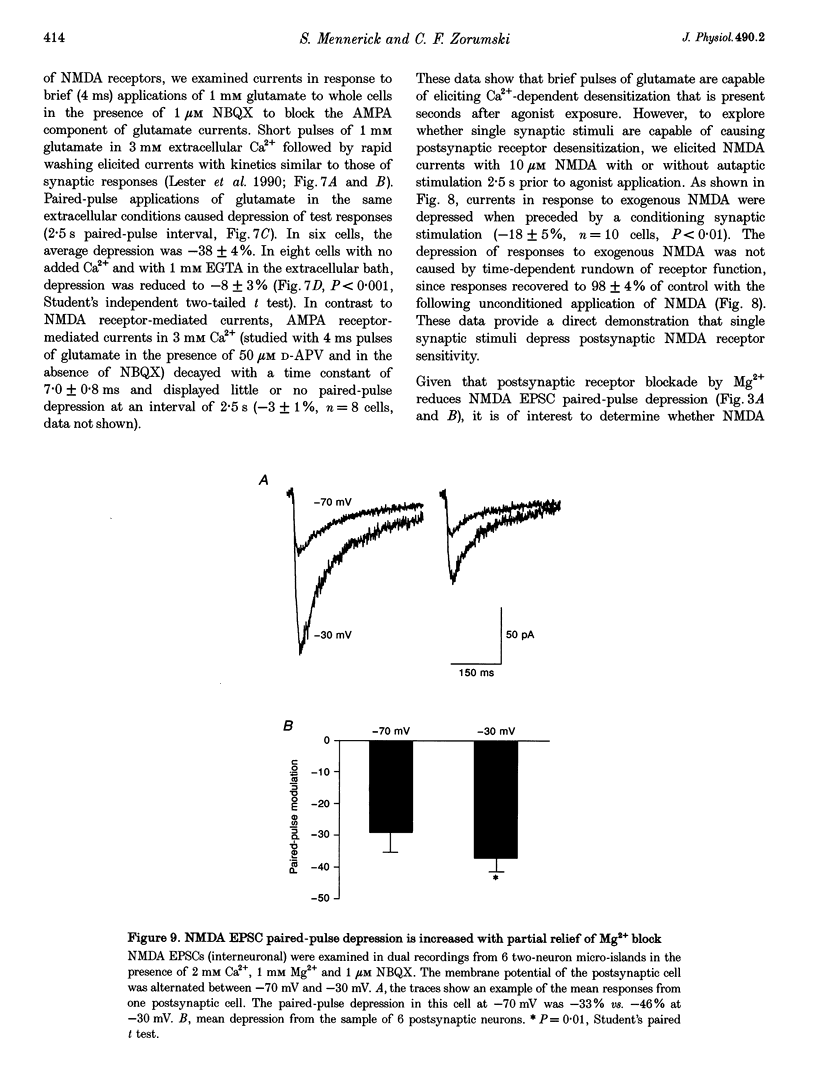
1. Paired-pulse synaptic stimulation of hippocampal neurons in microcultures resulted in depression of synaptic currents mediated by both NMDA and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors. However, NMDA EPSCs were more severely depressed than AMPA EPSCs. 2. Partial NMDA receptor blockade reduced paired-pulse depression of NMDA but not of AMPA synaptic currents while partial AMPA receptor blockade had no effect on paired-pulse depression of AMPA EPSCs. These results suggest that ion flux through NMDA receptors is important in paired-pulse depression of NMDA responses but has no effect on AMPA responses. 3. Low extracellular Ca2+ concentrations or positive postsynaptic holding potentials reduced paired-pulse depression of NMDA EPSCs to near that of AMPA responses. 4. Brief paired applications of exogenous glutamate to neurons produced Ca(2+)-dependent depression similar to the depression of NMDA synaptic responses and synaptic stimulation depressed responses to exogenously applied NMDA. 5. Physiological concentrations of Mg2+ prevented expression of the postsynaptic modulation of NMDA EPSCs at -70 mV, but partial relief of Mg2+ block of the NMDA channel with depolarization increased paired-pulse depression of NMDA EPSCs.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekkers J. M., Stevens C. F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Synergism at central synapses due to lateral diffusion of transmitter. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8708–8712. doi: 10.1073/pnas.85.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Different glutamate receptor channels mediate fast excitatory synaptic currents in inhibitory and excitatory cortical neurons. Neuron. 1993 Dec;11(6):1083–1091. doi: 10.1016/0896-6273(93)90221-c. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992 Sep;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992 Feb;12(2):635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980 Oct 13;199(1):1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- Mennerick S., Que J., Benz A., Zorumski C. F. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995 Jan;73(1):320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- Mennerick S., Zorumski C. F. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol. 1995 Oct 1;488(Pt 1):85–101. doi: 10.1113/jphysiol.1995.sp020948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S., Zorumski C. F. Presynaptic influence on the time course of fast excitatory synaptic currents in cultured hippocampal cells. J Neurosci. 1995 Apr;15(4):3178–3192. doi: 10.1523/JNEUROSCI.15-04-03178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. J., Nicoll R. A. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J Physiol. 1993 Nov;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff A., Lambert J. D. Factors contributing to the decay of the stimulus-evoked IPSC in rat hippocampal CA1 neurons. J Neurophysiol. 1994 Dec;72(6):2911–2926. doi: 10.1152/jn.1994.72.6.2911. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Feltz A., Westbrook G. L. Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1995 Jan;73(1):427–430. doi: 10.1152/jn.1995.73.1.427. [DOI] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Segal M. M., Furshpan E. J. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J Neurophysiol. 1990 Nov;64(5):1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958 Jun 15;8(2):102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- Thio L. L., Clark G. D., Clifford D. B., Zorumski C. F. Wheat germ agglutinin enhances EPSCs in cultured postnatal rat hippocampal neurons by blocking ionotropic quisqualate receptor desensitization. J Neurophysiol. 1992 Dec;68(6):1930–1938. doi: 10.1152/jn.1992.68.6.1930. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994 Jan;12(1):51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Tong G., Shepherd D., Jahr C. E. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995 Mar 10;267(5203):1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Zhang S., Raman I. M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993 Jun;10(6):1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Vyklicky L., Jr, Patneau D. K., Mayer M. L. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron. 1991 Dec;7(6):971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr Calcium-mediated modulation of N-methyl-D-aspartate (NMDA) responses in cultured rat hippocampal neurones. J Physiol. 1993 Oct;470:575–600. doi: 10.1113/jphysiol.1993.sp019876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J., Fischbach G. D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Mol Neurobiol. 1989 Mar;9(1):95–104. doi: 10.1007/BF00711446. [DOI] [PubMed] [Google Scholar]


