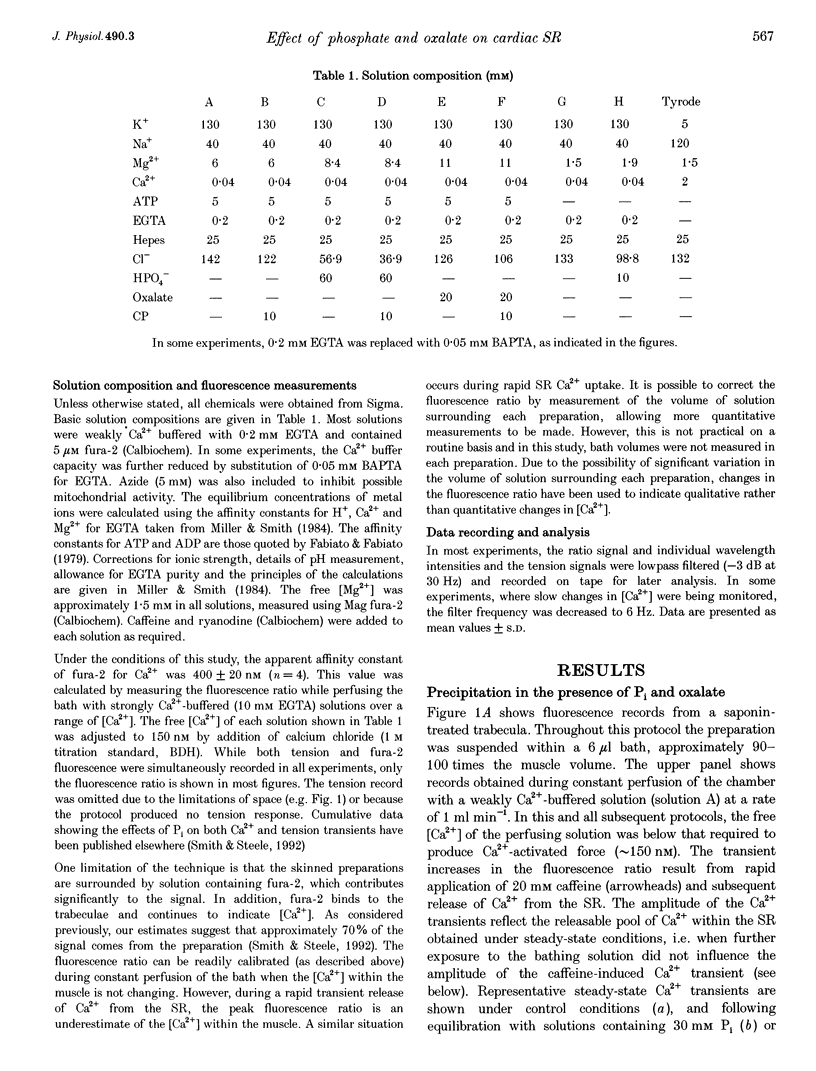
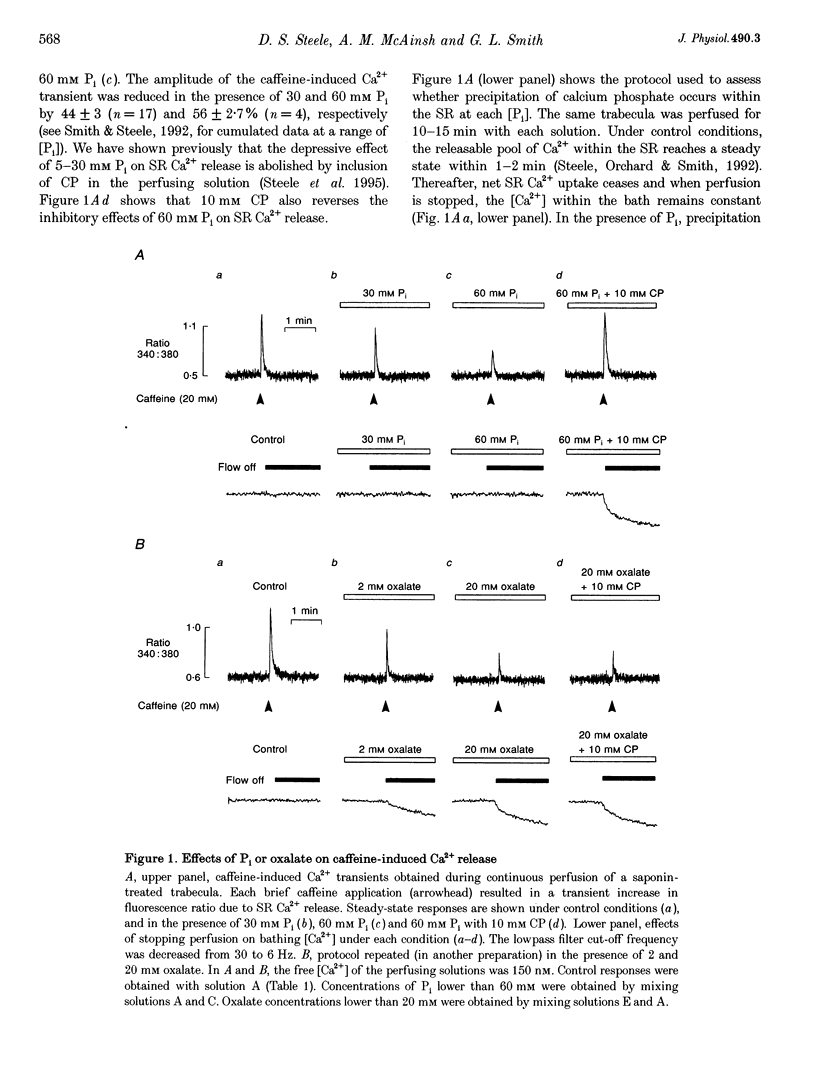
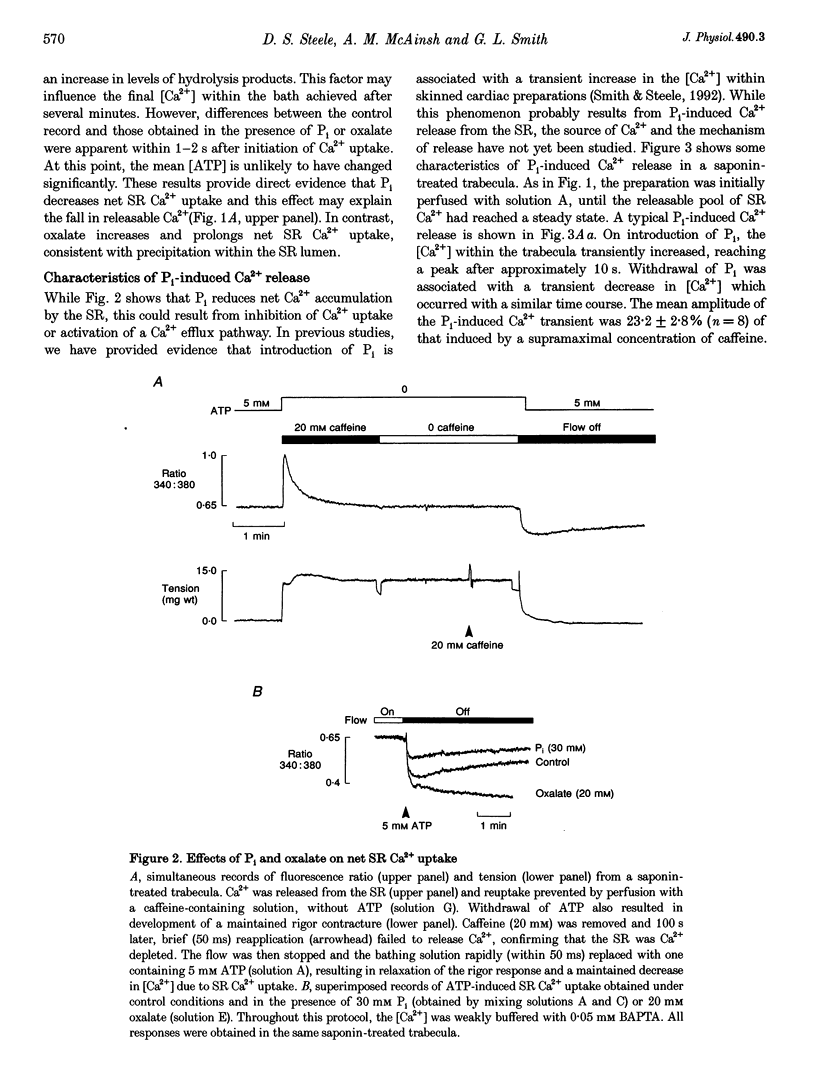
Abstract
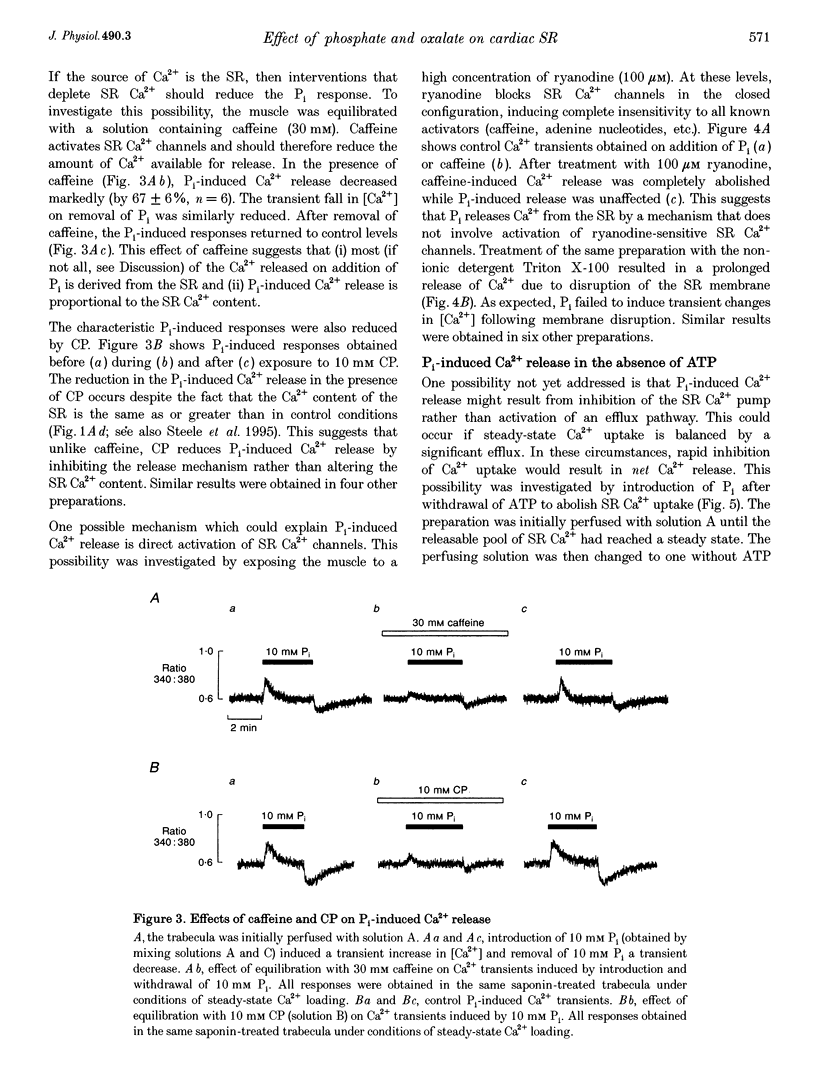
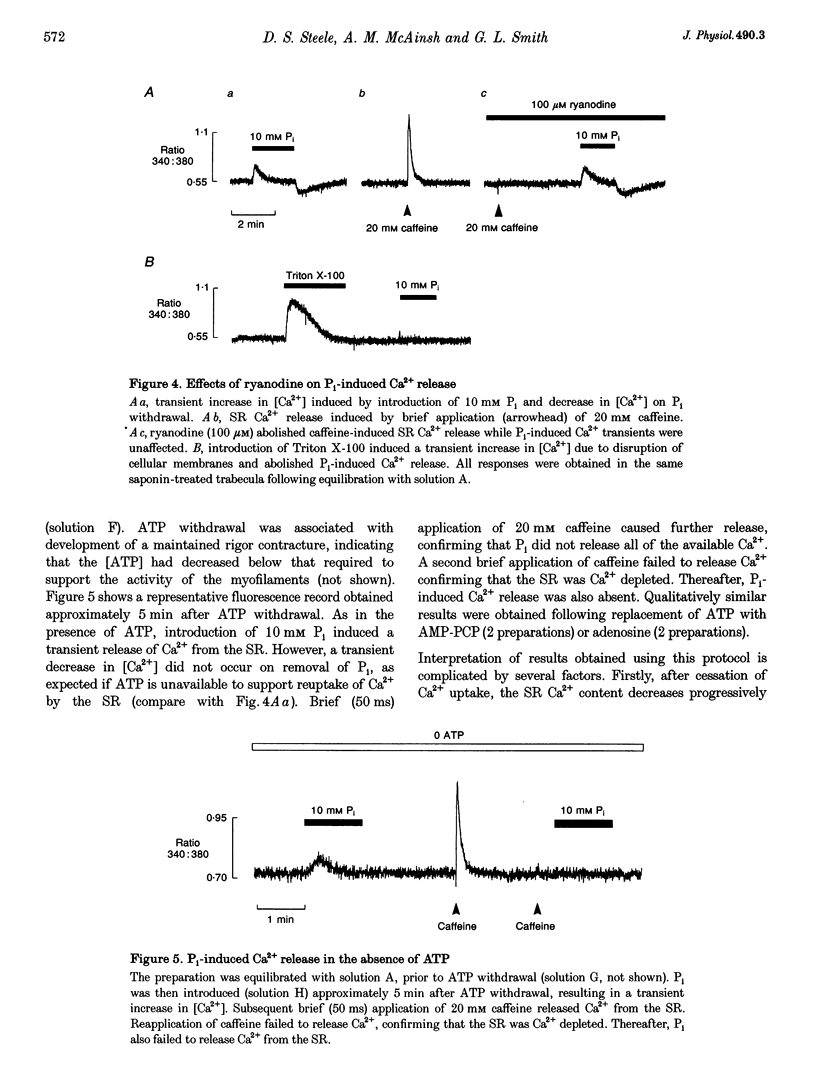
1. Ventricular trabeculae from the right ventricle of rat heart were suspended in a 6 microliters bath and "skinned' with saponin (50 mg ml-1). Preparations were perfused with solutions mimicking the intracellular milieu and the [Ca2+] within the bath was monitored continuously using fura-2. 2. Application of 20 mM caffeine released Ca2+ from the sarcoplasmic reticulum (SR), resulting in a transient increase in the fura-2 fluorescence ratio. Caffeine-induced Ca2+ transients were smaller in the presence of 30 or 60 mM inorganic phosphate (Pi). This depressive effect of Pi on SR function was reversed by 10 mM creatine phosphate (CP). Caffeine-induced Ca2+ transients were also reduced in the presence of 10 mM oxalate, although this effect was not reversed by CP. 3. When perfusion was stopped in the presence of 30 or 60 mM Pi, the [Ca2+] within the bath remained constant. However, when the flow was stopped in the presence of 60 mM Pi and 10 mM CP, a prolonged decrease in [Ca2+] occurred, consistent with precipitation of calcium phosphate within the SR. A similar decrease in [Ca2+] was observed when perfusion was stopped in the presence of 2 or 20 mM oxalate, in the absence or presence of CP. 4. The SR was Ca2+ depleted by withdrawal of ATP and exposure to 20 mM caffeine. Perfusion was then stopped and ATP reapplied, resulting in a maintained decrease in [Ca2+] within the bath, due to SR Ca2+ uptake. Net Ca2+ uptake was markedly reduced in the presence of 30 mM Pi. In contrast, 20 mM oxalate increased Ca2+ uptake and the [Ca2+] within the bath continued to fall over 2-3 min. 5. Introduction of Pi released Ca2+ from the SR. Ryanodine (100 microM) abolished caffeine-induced Ca2+ release while Pi-induced Ca2+ release was unaffected. Pi-induced Ca2+ release was reduced in the constant presence of 20 mM caffeine or 10 mM CP and was abolished completely by disruption of the SR membrane with Triton X-100. Pi-induced Ca2+ release occurred after abolition of SR Ca2+ uptake by ATP withdrawal. 6. These results suggest that the Pi-induced decrease in releasable Ca2+ does not result from precipitation of calcium phosphate within the SR lumen. Pi inhibits net SR Ca2+ uptake, but this appears to result from activation of a ryanodine-insensitive Ca2+ efflux pathway rather then inhibition of Ca2+ uptake. Possible mechanisms are considered, including reversal of the SR Ca2+ pump.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Baskin R. J. ATP synthesis in sarcoplasmic reticulum. Arch Biochem Biophys. 1972 Nov;153(1):47–54. doi: 10.1016/0003-9861(72)90418-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Effects of ryanodine in skinned cardiac cells. Fed Proc. 1985 Dec;44(15):2970–2976. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Feher J. J., Lipford G. B. Calcium oxalate and calcium phosphate capacities of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Sep 10;818(3):373–385. doi: 10.1016/0005-2736(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Fruen B. R., Mickelson J. R., Shomer N. H., Roghair T. J., Louis C. F. Regulation of the sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. J Biol Chem. 1994 Jan 7;269(1):192–198. [PubMed] [Google Scholar]
- Fryer M. W., Owen V. J., Lamb G. D., Stephenson D. G. Effects of creatine phosphate and P(i) on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J Physiol. 1995 Jan 1;482(Pt 1):123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W. The reversibility of the sarcoplasmic calcium pump. Biochim Biophys Acta. 1978 Apr 10;515(1):23–53. doi: 10.1016/0304-4157(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Inesi G., de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989 Apr 5;264(10):5929–5936. [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. ATP synthesis by the reverse of the sarcoplasmic calcium pump. FEBS Lett. 1971 Jan 30;12(5):271–272. doi: 10.1016/0014-5793(71)80196-5. [DOI] [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. Der Einfluss von Oxalat auf den Calcium-Transport isolierter Vesikel des sarkoplasmatischen Reticulum. Biochem Z. 1965 Dec 31;343(4):360–382. [PubMed] [Google Scholar]
- Miller D. J., Smith G. L. EGTA purity and the buffering of calcium ions in physiological solutions. Am J Physiol. 1984 Jan;246(1 Pt 1):C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Steele D. S. Inorganic phosphate decreases the Ca2+ content of the sarcoplasmic reticulum in saponin-treated rat cardiac trabeculae. J Physiol. 1992 Dec;458:457–473. doi: 10.1113/jphysiol.1992.sp019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler F., Teruel J. A., Fernandez-Belda F., Gomez-Fernandez J. C. Characterization of the steady-state calcium fluxes in skeletal sarcoplasmic reticulum vesicles. Role of the Ca2+ pump. Eur J Biochem. 1990 Sep 11;192(2):347–354. doi: 10.1111/j.1432-1033.1990.tb19233.x. [DOI] [PubMed] [Google Scholar]
- Steele D. S., McAinsh A. M., Smith G. L. Effects of creatine phosphate and inorganic phosphate on the sarcoplasmic reticulum of saponin-treated rat heart. J Physiol. 1995 Feb 15;483(Pt 1):155–166. doi: 10.1113/jphysiol.1995.sp020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen G. J., van Graas I. A., Elzinga G. Uptake and caffeine-induced release of calcium in fast muscle fibers of Xenopus laevis: effects of MgATP and P(i). Am J Physiol. 1993 Sep;265(3 Pt 1):C650–C657. doi: 10.1152/ajpcell.1993.265.3.C650. [DOI] [PubMed] [Google Scholar]
- Suko J., Hellmann G., Winkler F. The reversal of the calcium pump of cardiac sarcoplasmic reticulum. Basic Res Cardiol. 1977 Mar-Jun;72(2-3):147–152. doi: 10.1007/BF01906353. [DOI] [PubMed] [Google Scholar]
- Winkler F., Suko J. Phosphorylation of the calcium-transport adenosine triphosphate of cardiac sarcoplasmic reticulum by orthophosphate. Eur J Biochem. 1977 Aug 1;77(3):611–619. doi: 10.1111/j.1432-1033.1977.tb11705.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Nosek T. M. Intracellular milieu changes associated with hypoxia impair sarcoplasmic reticulum Ca2+ transport in cardiac muscle. Am J Physiol. 1991 Sep;261(3 Pt 2):H620–H626. doi: 10.1152/ajpheart.1991.261.3.H620. [DOI] [PubMed] [Google Scholar]