Abstract
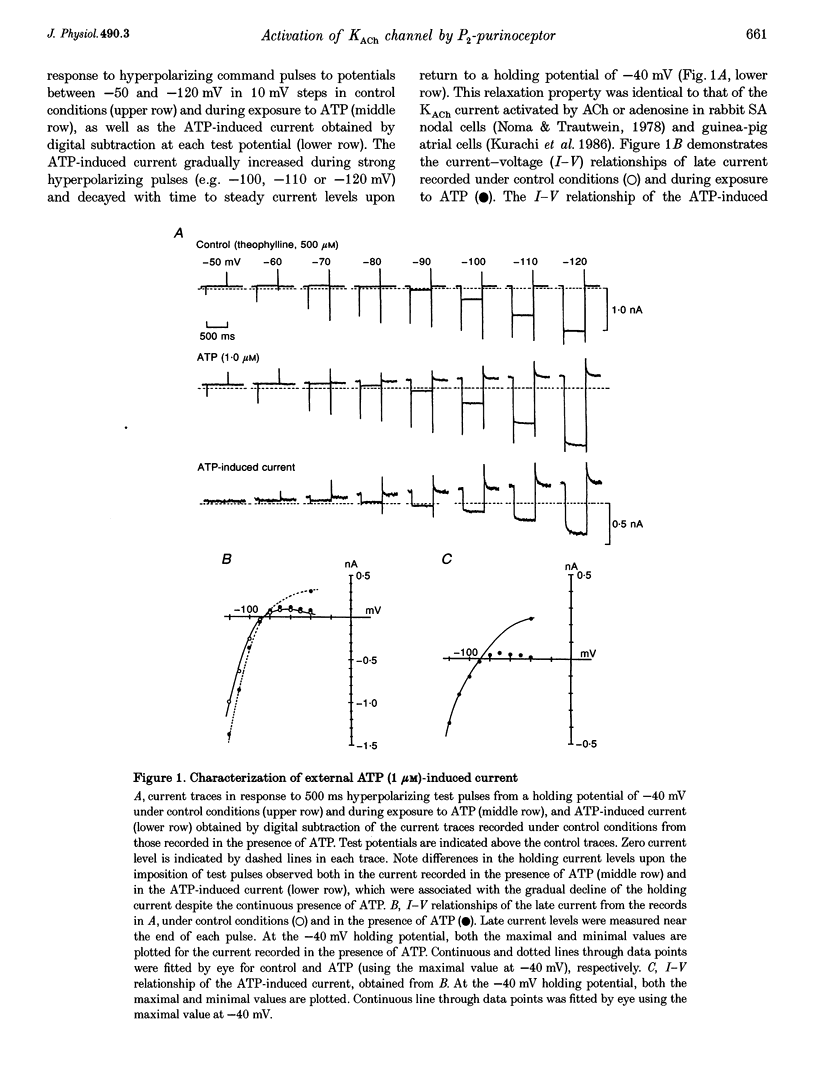
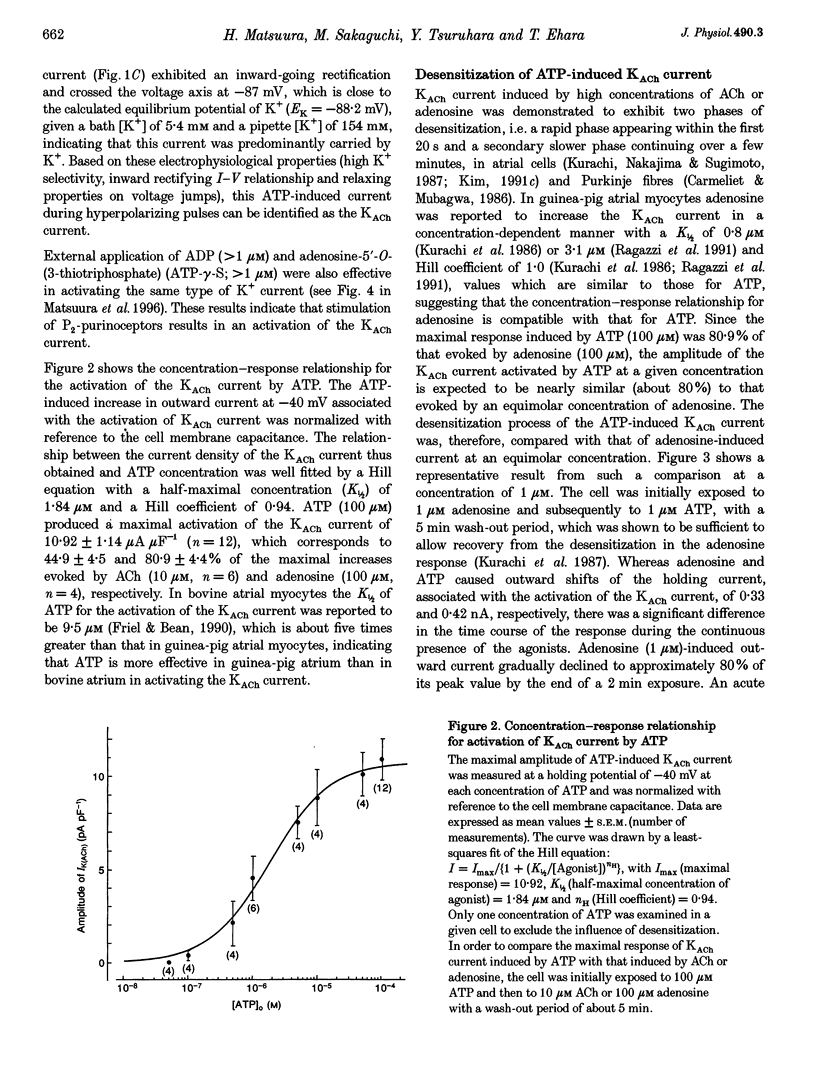
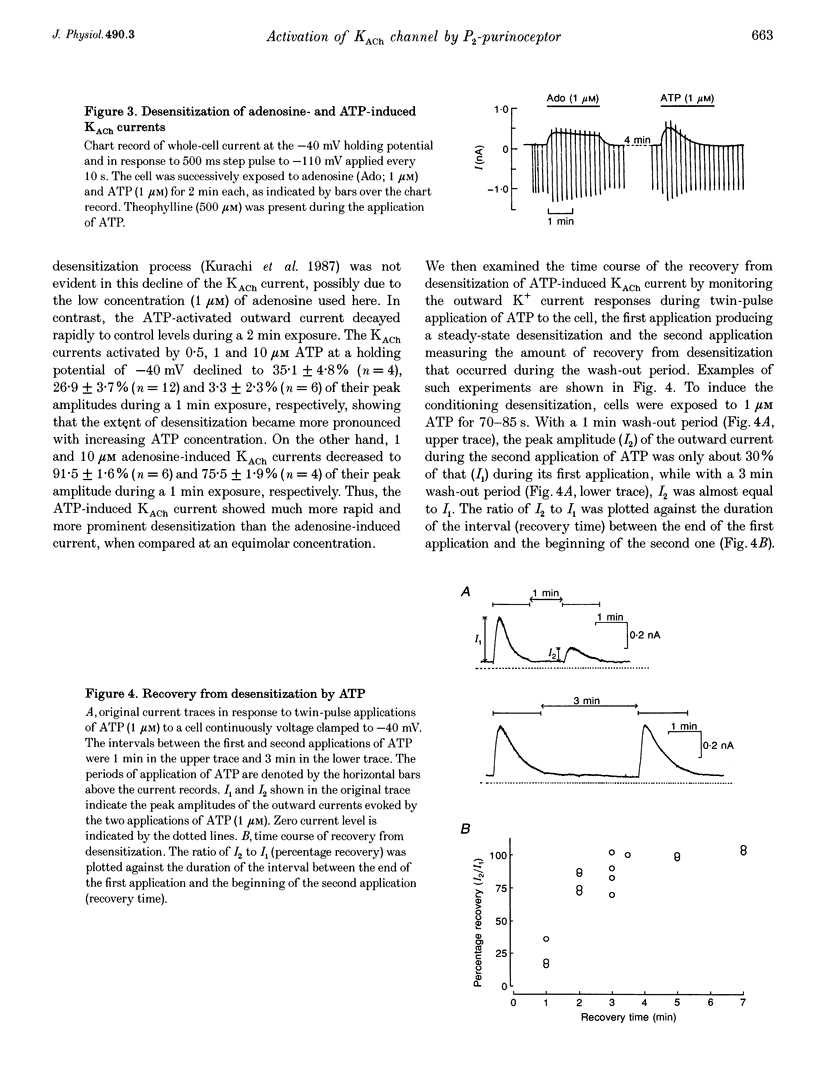
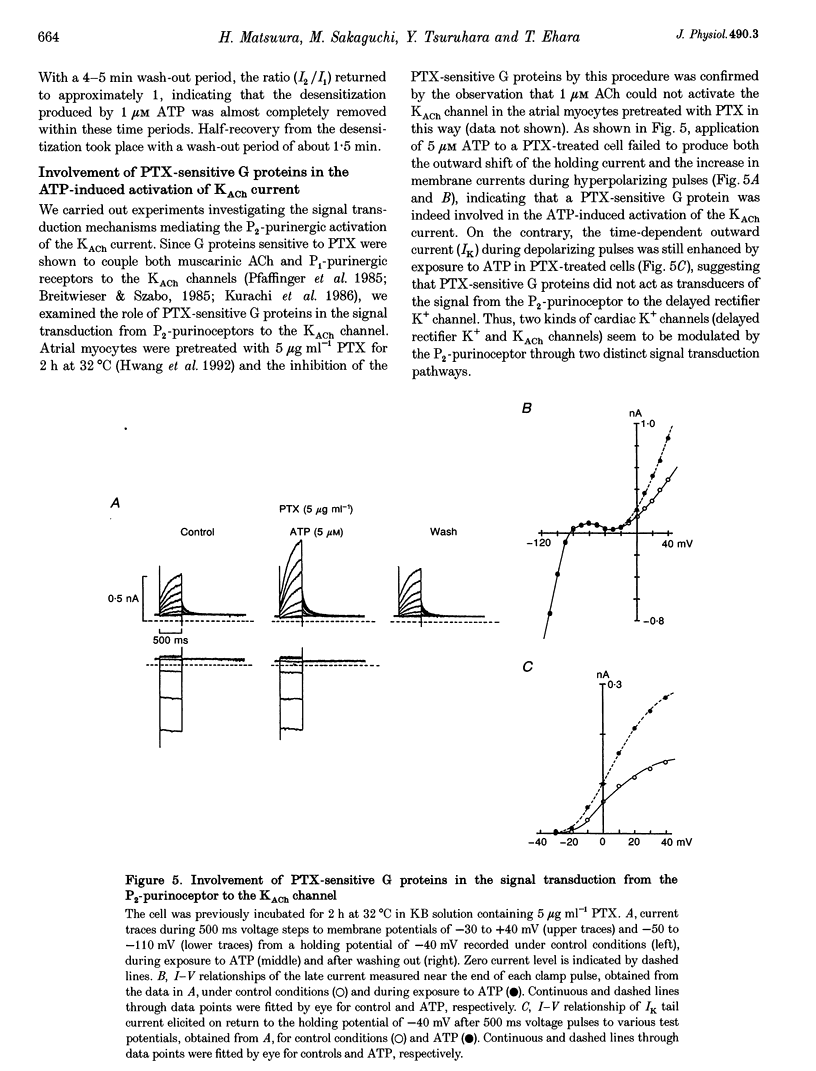
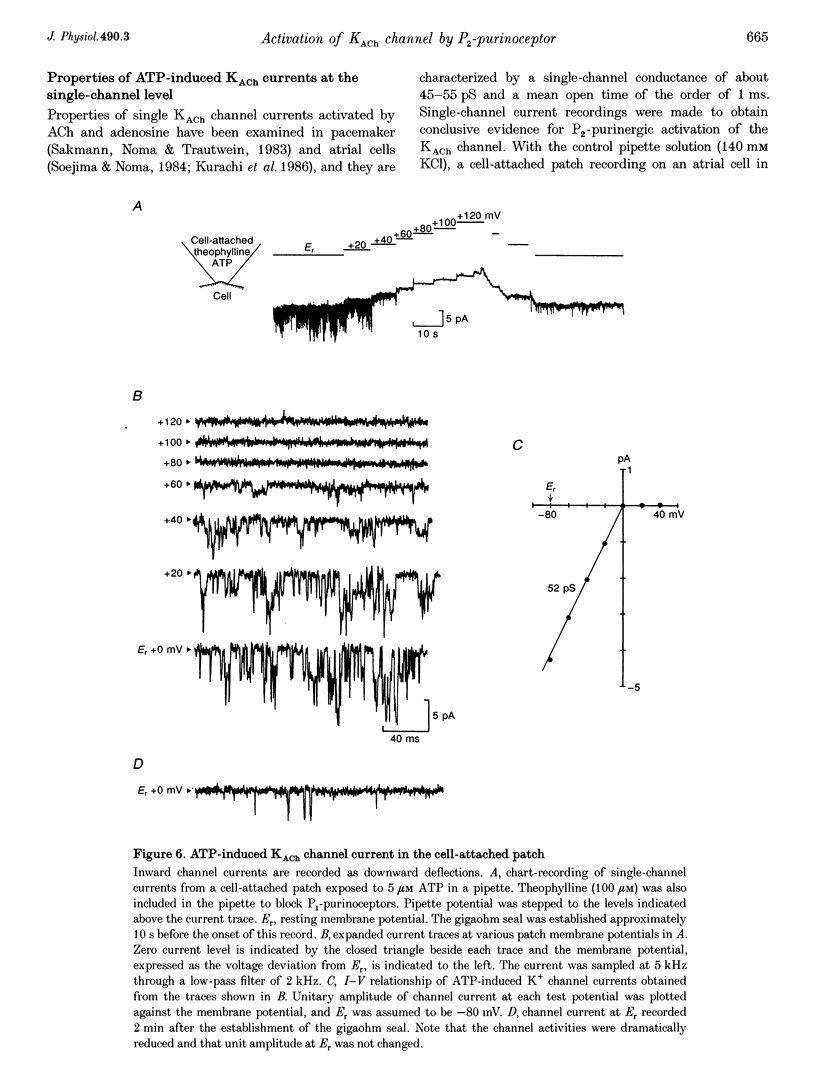
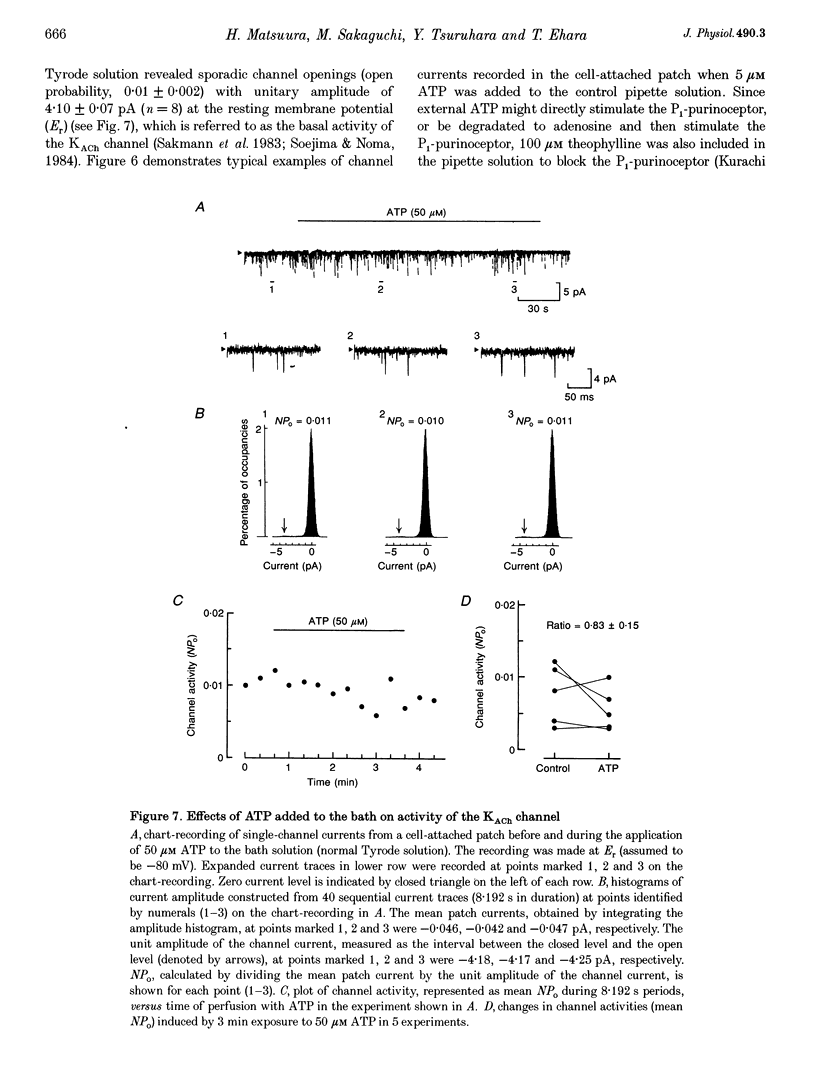
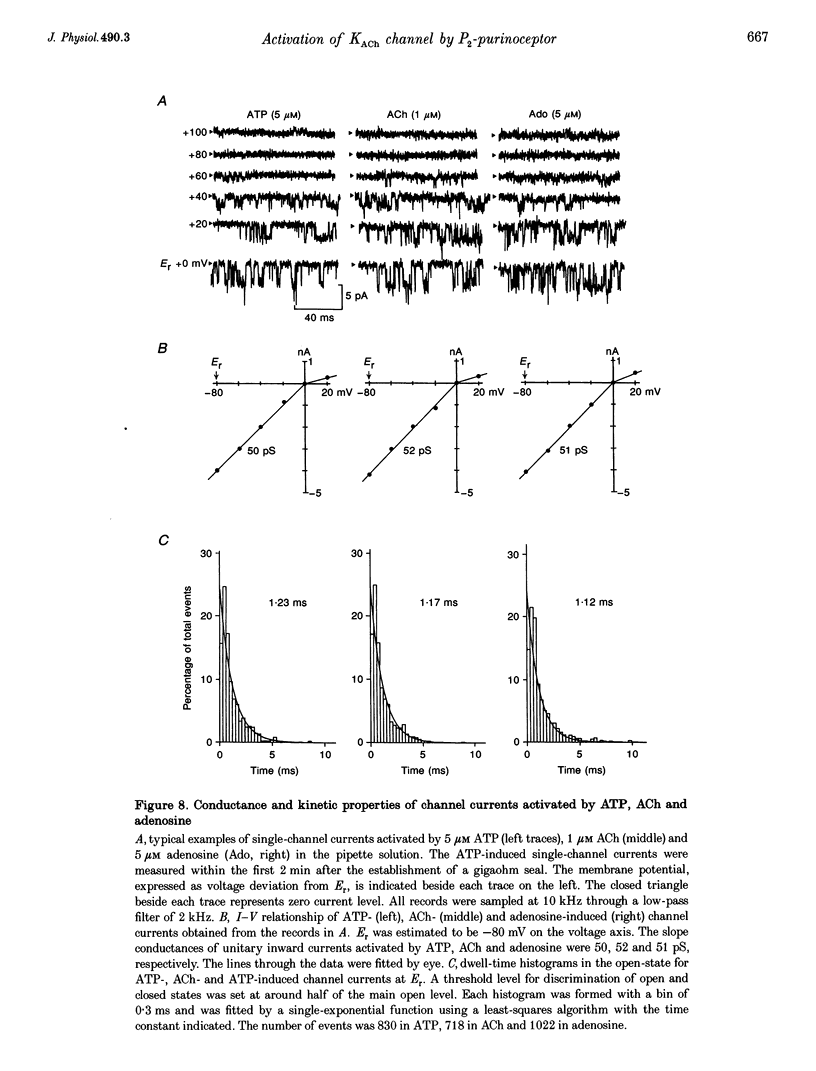
1. Whole-cell voltage clamp and cell-attached patch-clamp techniques were applied to single atrial myocytes enzymatically dissociated from adult guinea-pig hearts. 2. In whole-cell clamp conditions, external applications, of ATP activated the muscarinic K+ (KACh) current, identified by its inward rectification, its reversal potential near the calculated K+ equilibrium potential (EK) and its relaxation properties during step changes of whole-cell membrane potential. Theophylline, an antagonist for Pi-purinoceptors, did not affect the action of ATP on the KACh current, indicating that the response was evoked through P2-purinoceptors. 3. The concentration-response relationship for ATP was well described by a Hill equation with a half-maximal concentration of 1.84 microM and a Hill coefficient of 0.94. ATP (100 microM) produced a maximal increase of the KACh current to 10.92 microA microF-1, which corresponds to 44.9 and 80.9% of the maximal increases evoked by ACh (10 microM) and adenosine (100 microM), respectively. 4. The activation of KACh current gradually declined to a steady level despite the continuous presence of ATP (desensitization). Recovery from the desensitization was relatively rapid with a half-time of approximately 1.5 min. 5. The activation of KACh current by ATP was completely abolished by pre-incubating myocytes with pertussis toxin (PTX, 5 micrograms ml-1), indicating that P2-purinoceptors are coupled to PTX-sensitive G proteins to activate the KACh channel. 6. In the cell-attached patch recording, ATP (5 microM) applied to the pipette solution enhanced the activity of a channel with single-channel conductance of 52.7 +/- 0.9 pS (mean +/- S.E.M., n = 10), reversal potential near EK and mean open time of 1.1 +/- 0.1 ms. These conductance and kinetic properties are identical to those of the KACh channel in the heart. In contrast, ATP applied to the bath solution did not significantly affect the basal activity of KACh channel openings. These observations suggest that the mechanism coupling the P2-purinoceptor to the activation of the KACh channel involves membrane-delimited component(s) rather than soluble second messenger(s). 7. These results strongly suggest a direct coupling of the P2-purinoceptor to the KACh channel through PTX-sensitive G proteins, analogous to the coupling mechanism of the muscarinic ACh receptor and Pi-purinoceptor to this channel.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banach K., Hüser J., Lipp P., Wellner M. C., Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by a serum factor. J Physiol. 1993 Feb;461:263–281. doi: 10.1113/jphysiol.1993.sp019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983 May;244(5):H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Shryock J., West G. A., Clemo H. F., DiMarco J. P., Berne R. M. Effects of adenosine and adenine nucleotides on the atrioventricular node of isolated guinea pig hearts. Circulation. 1984 Dec;70(6):1083–1091. doi: 10.1161/01.cir.70.6.1083. [DOI] [PubMed] [Google Scholar]
- Belhassen B., Glick A., Laniado S. Comparative clinical and electrophysiologic effects of adenosine triphosphate and verapamil on paroxysmal reciprocating junctional tachycardia. Circulation. 1988 Apr;77(4):795–805. doi: 10.1161/01.cir.77.4.795. [DOI] [PubMed] [Google Scholar]
- Born G. V., Kratzer M. A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol. 1984 Sep;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Bünemann M., Pott L. Membrane-delimited activation of muscarinic K current by an albumin-associated factor in guinea-pig atrial myocytes. Pflugers Arch. 1993 Nov;425(3-4):329–334. doi: 10.1007/BF00374183. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Desensitization of the acetylcholine-induced increase of potassium conductance in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:239–255. doi: 10.1113/jphysiol.1986.sp015971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E., Klöckner U., Isenberg G. The alpha subunit of the GTP binding protein activates muscarinic potassium channels of the atrium. Science. 1988 Jun 24;240(4860):1782–1783. doi: 10.1126/science.2454511. [DOI] [PubMed] [Google Scholar]
- Clemens M. G., Forrester T. Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol. 1981 Mar;312:143–158. doi: 10.1113/jphysiol.1981.sp013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Dual control by ATP and acetylcholine of inwardly rectifying K+ channels in bovine atrial cells. Pflugers Arch. 1990 Mar;415(6):651–657. doi: 10.1007/BF02584001. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Nairn A. C., Gadsby D. C. Role of GTP-binding proteins in the regulation of mammalian cardiac chloride conductance. J Gen Physiol. 1992 Apr;99(4):465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kim D. Calcitonin-gene-related peptide activates the muscarinic-gated K+ current in atrial cells. Pflugers Arch. 1991 May;418(4):338–345. doi: 10.1007/BF00550871. [DOI] [PubMed] [Google Scholar]
- Kim D. Endothelin activation of an inwardly rectifying K+ current in atrial cells. Circ Res. 1991 Jul;69(1):250–255. doi: 10.1161/01.res.69.1.250. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Kim D. Modulation of acetylcholine-activated K+ channel function in rat atrial cells by phosphorylation. J Physiol. 1991 Jun;437:133–155. doi: 10.1113/jphysiol.1991.sp018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Alpha-adrenergic activation of the muscarinic K+ channel is mediated by arachidonic acid metabolites. Pflugers Arch. 1989 May;414(1):102–104. doi: 10.1007/BF00585635. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Clapham D. E. Somatostatin activates an inwardly rectifying K+ channel in neonatal rat atrial cells. Pflugers Arch. 1989 Aug;414(4):492–494. doi: 10.1007/BF00585062. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Martin M. W., Harden T. K. Agonist-induced desensitization of a P2Y-purinergic receptor-regulated phospholipase C. J Biol Chem. 1989 Nov 25;264(33):19535–19539. [PubMed] [Google Scholar]
- Matsuura H., Tsuruhara Y., Sakaguchi M., Ehara T. Enhancement of delayed rectifier K+ current by P2-purinoceptor stimulation in guinea-pig atrial cells. J Physiol. 1996 Feb 1;490(Pt 3):647–658. doi: 10.1113/jphysiol.1996.sp021174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Sugimoto T., Kurachi Y. Platelet-activating factor activates cardiac GK via arachidonic acid metabolites. FEBS Lett. 1991 Sep 9;289(2):239–243. doi: 10.1016/0014-5793(91)81079-n. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Ono K., Tsujimoto G., Sakamoto A., Eto K., Masaki T., Ozaki Y., Satake M. Endothelin-A receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature. 1994 Jul 28;370(6487):301–304. doi: 10.1038/370301a0. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzi E., Wu S. N., Shryock J., Belardinelli L. Electrophysiological and receptor binding studies to assess activation of the cardiac adenosine receptor by adenine nucleotides. Circ Res. 1991 Apr;68(4):1035–1044. doi: 10.1161/01.res.68.4.1035. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J., Lo C. F., Breitwieser G. E. Platelet-activating factor receptor-dependent activation of the muscarinic K+ current in bullfrog atrial myocytes. Circ Res. 1993 Apr;72(4):786–794. doi: 10.1161/01.res.72.4.786. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Pinkas R., Raz A. Evidence for different purinergic receptors for ATP and ADP in rabbit kidney and heart. Eur J Pharmacol. 1981 Sep 11;74(2-3):167–173. doi: 10.1016/0014-2999(81)90527-6. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Takikawa R., Kurachi Y., Mashima S., Sugimoto T. Adenosine-5'-triphosphate-induced sinus tachycardia mediated by prostaglandin synthesis via phospholipase C in the rabbit heart. Pflugers Arch. 1990 Sep;417(1):13–20. doi: 10.1007/BF00370763. [DOI] [PubMed] [Google Scholar]


