Abstract
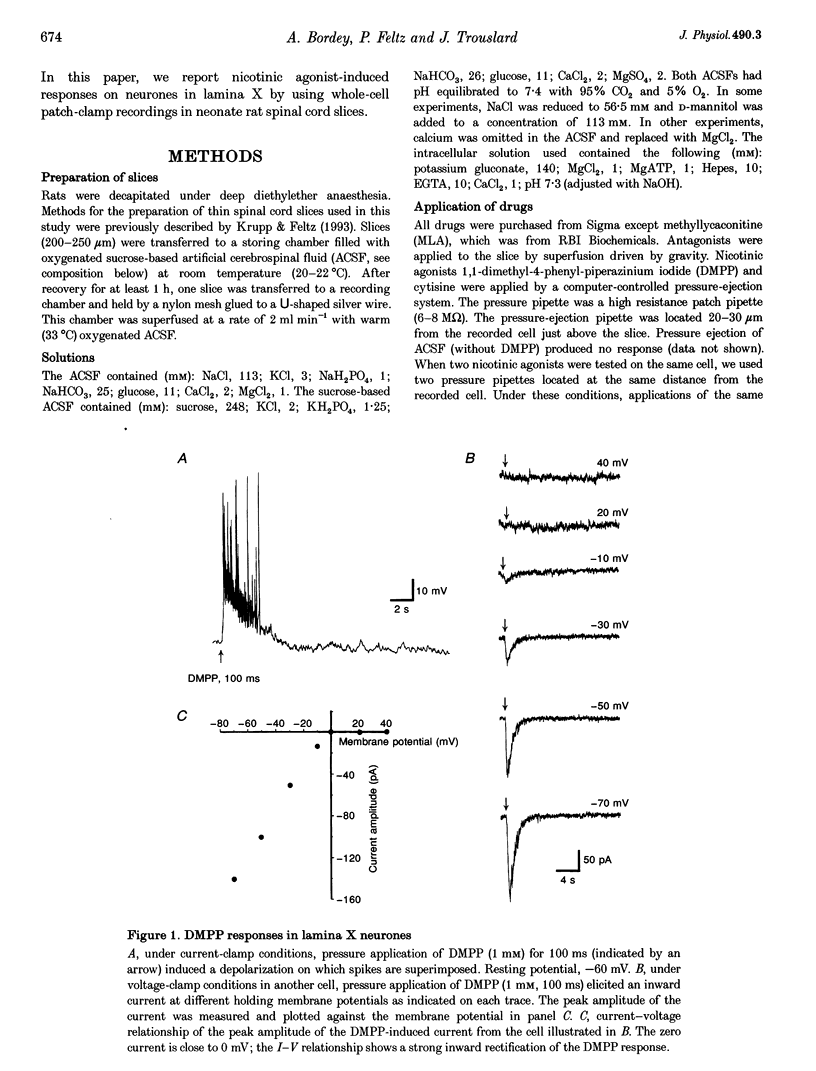
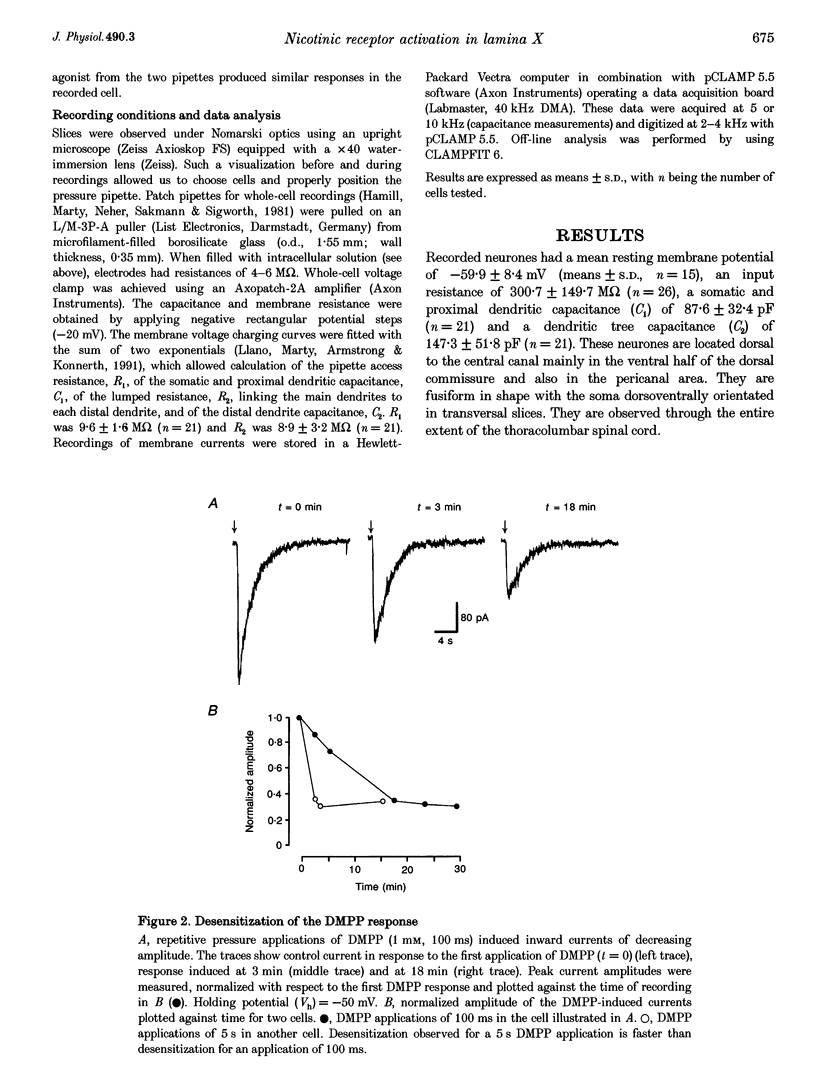
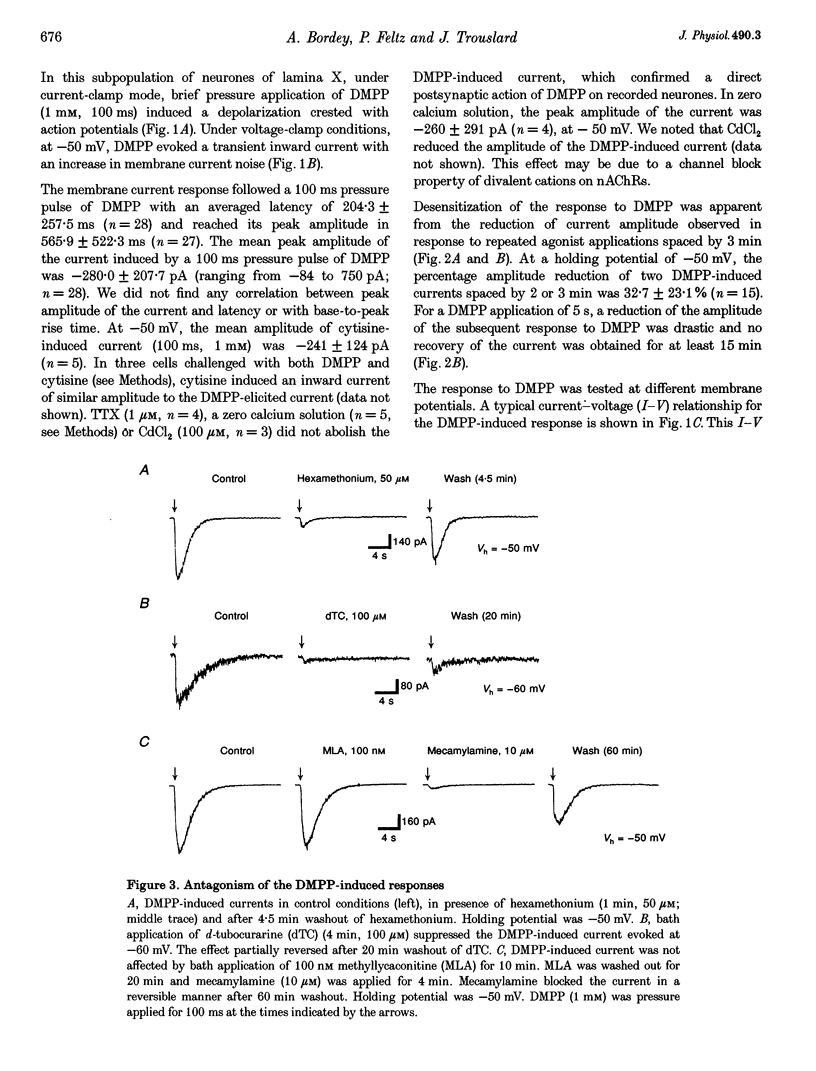
1. Nicotinic acetylcholine receptors (nAChRs) on lamina X neurones in neonate (P1-P12) rat transverse thoracolumbar spinal cord slices were studied using the whole-cell patch-clamp technique. These visually selected neurones are located dorsal to the central canal, mainly in the ventral half of the dorsal commissure. 2. Pressure application of the nicotinic agonist 1,1-dimethyl-4-phenyl-piperazinium (DMPP) (1 mM) induced a rapid depolarization on which action potentials are superimposed. 3. At -50 mV, DMPP (1 mM), pressure ejected for 100 ms, induced a fast inward current with a mean amplitude of -280 pA (n = 28) in 90% of the neurones recorded. Superfusion of tetrodotoxin (TTX), a solution containing 0 Ca(2+)-high Mg2+, CdCl2 or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) did not abolish the DMPP-induced current, which confirmed a direct postsynaptic effect of DMPP on recorded neurones. 3. The current-voltage (I-V) relationship for DMPP-induced current exhibited a reversal potential of 0 mV (NaCl outside, potassium gluconate inside) and a strong inward rectification. 4. The DMPP-induced responses were blocked by mecamylamine, hexamethonium and d-tubocurarine (dTC) but were insensitive to alpha-bungarotoxin and methyllycaconitine (MLA). 5. We conclude that lamina X neurones located dorsally to the central canal possess nicotinic acetylcholine receptors. Activation of these nicotinic receptors results in depolarization and generation of action potentials. These receptors may be involved in the modulation of the somato- and viscerosensory transmission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkondon M., Albuquerque E. X. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993 Jun;265(3):1455–1473. [PubMed] [Google Scholar]
- Alkondon M., Pereira E. F., Wonnacott S., Albuquerque E. X. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992 Apr;41(4):802–808. [PubMed] [Google Scholar]
- Barber R. P., Phelps P. E., Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984 Nov 1;229(3):329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Borges L. F., Iversen S. D. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Res. 1986 Jan 1;362(1):140–148. doi: 10.1016/0006-8993(86)91407-1. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The acetylcholine receptors of Renshaw cells. Exp Brain Res. 1966;2(1):66–80. doi: 10.1007/BF00234361. [DOI] [PubMed] [Google Scholar]
- Dineley-Miller K., Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, beta4, are distributed in multiple areas of the rat central nervous system. Brain Res Mol Brain Res. 1992 Dec;16(3-4):339–344. doi: 10.1016/0169-328x(92)90244-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hochman S., Jordan L. M., MacDonald J. F. N-methyl-D-aspartate receptor-mediated voltage oscillations in neurons surrounding the central canal in slices of rat spinal cord. J Neurophysiol. 1994 Aug;72(2):565–577. doi: 10.1152/jn.1994.72.2.565. [DOI] [PubMed] [Google Scholar]
- Hosoya Y., Nadelhaft I., Wang D., Kohno K. Thoracolumbar sympathetic preganglionic neurons in the dorsal commissural nucleus of the male rat: an immunohistochemical study using retrograde labeling of cholera toxin subunit B. Exp Brain Res. 1994;98(1):21–30. doi: 10.1007/BF00229105. [DOI] [PubMed] [Google Scholar]
- Khan I. M., Taylor P., Yaksh T. L. Cardiovascular and behavioral responses to nicotinic agents administered intrathecally. J Pharmacol Exp Ther. 1994 Jul;270(1):150–158. [PubMed] [Google Scholar]
- Khan I. M., Yaksh T. L., Taylor P. Ligand specificity of nicotinic acetylcholine receptors in rat spinal cord: studies with nicotine and cytisine. J Pharmacol Exp Ther. 1994 Jul;270(1):159–166. [PubMed] [Google Scholar]
- Krupp J., Feltz P. Synaptic- and agonist-induced chloride currents in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1993 Nov;471:729–748. doi: 10.1113/jphysiol.1993.sp019925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte C. C. Lamina X of primate spinal cord: distribution of five neuropeptides and serotonin. Neuroscience. 1988 May;25(2):639–658. doi: 10.1016/0306-4522(88)90265-5. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslinski N. R., Randić M. Responses of identified spinal neurones to acetylcholine applied by micro-electrophoresis. J Physiol. 1977 Jul;269(1):195–219. doi: 10.1113/jphysiol.1977.sp011899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin R. L., Madsen A. M., Giesler G. J., Jr Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J Comp Neurol. 1983 Nov 1;220(3):321–335. doi: 10.1002/cne.902200306. [DOI] [PubMed] [Google Scholar]
- Perrins R., Roberts A. Nicotinic and muscarinic ACh receptors in rhythmically active spinal neurones in the Xenopus laevis embryo. J Physiol. 1994 Jul 15;478(Pt 2):221–228. doi: 10.1113/jphysiol.1994.sp020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role L. W. Diversity in primary structure and function of neuronal nicotinic acetylcholine receptor channels. Curr Opin Neurobiol. 1992 Jun;2(3):254–262. doi: 10.1016/0959-4388(92)90112-x. [DOI] [PubMed] [Google Scholar]
- Séguéla P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993 Feb;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]


