Abstract
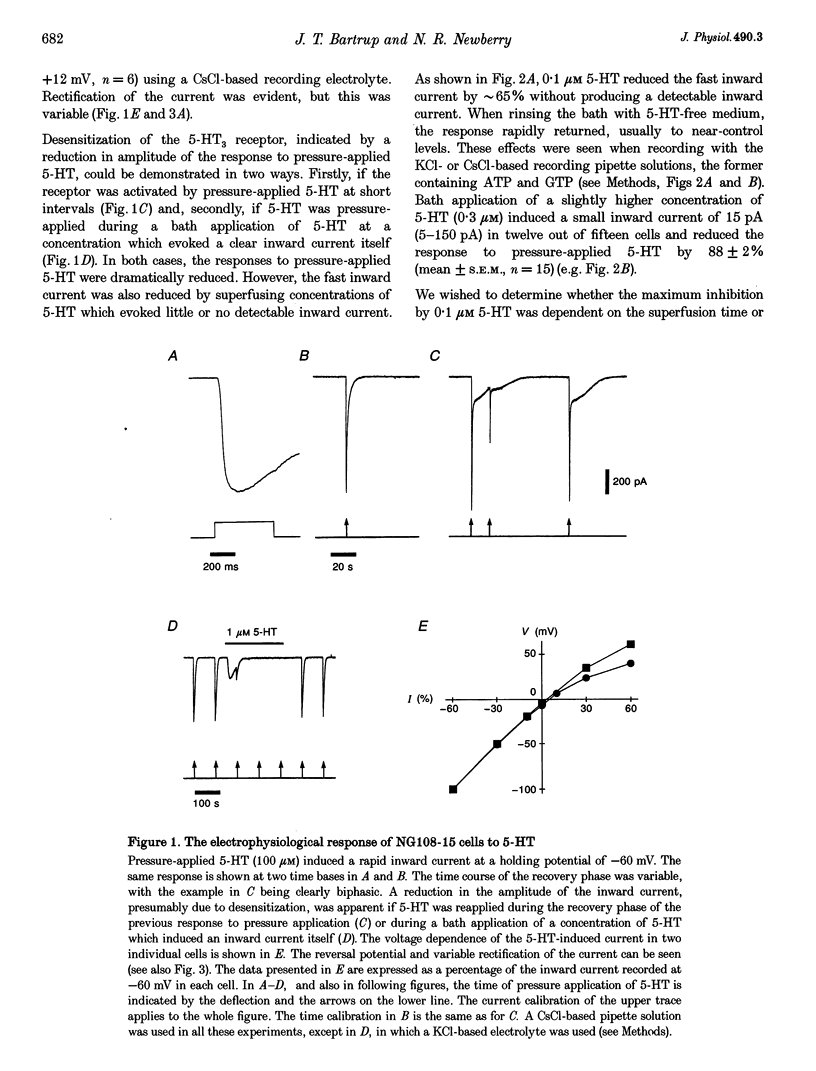
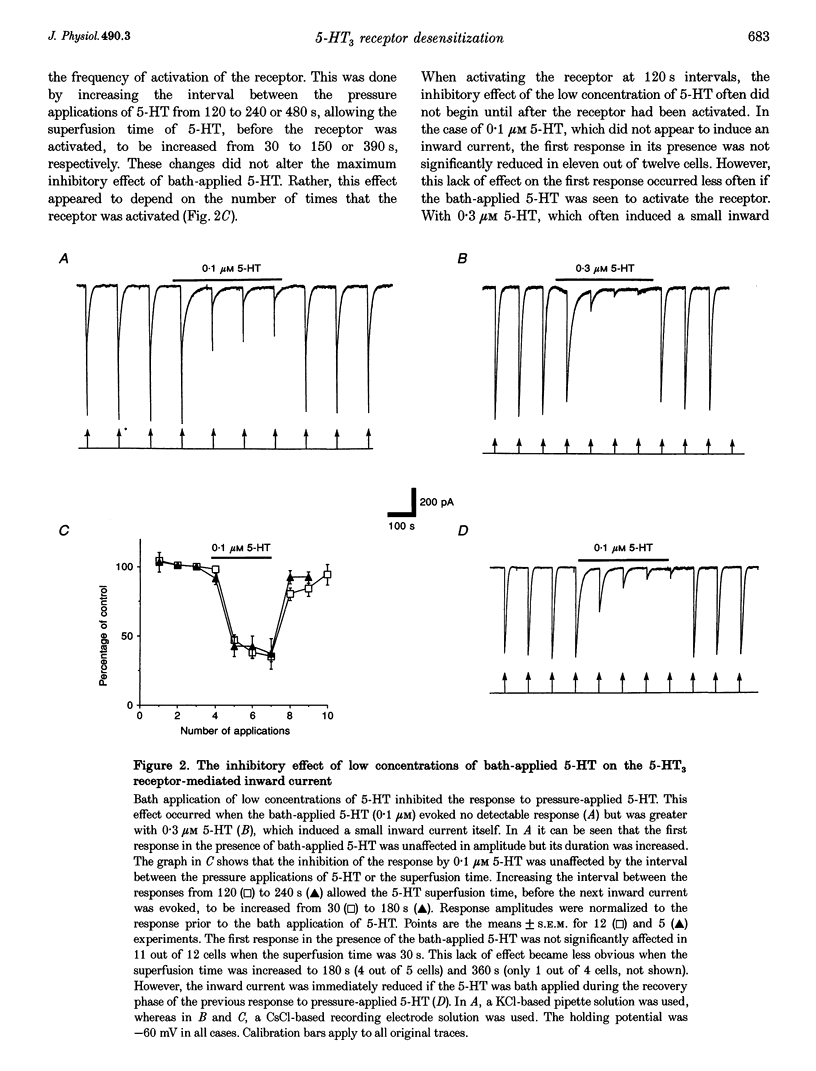
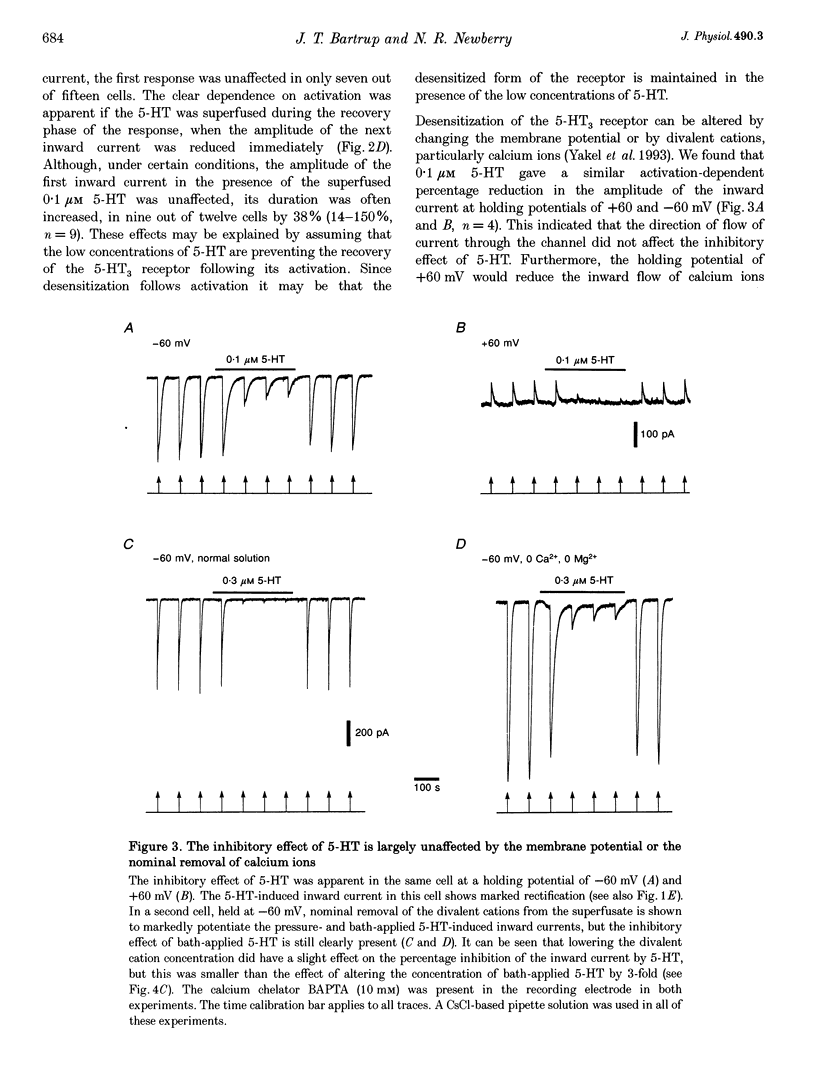
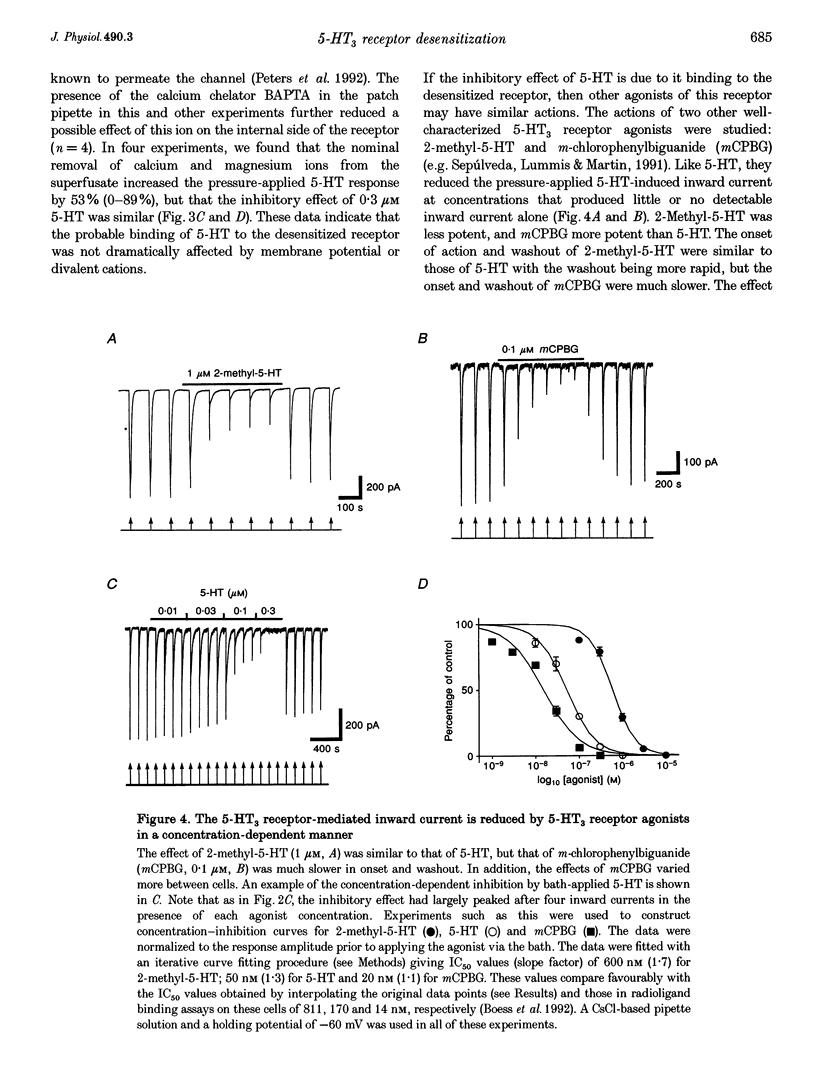
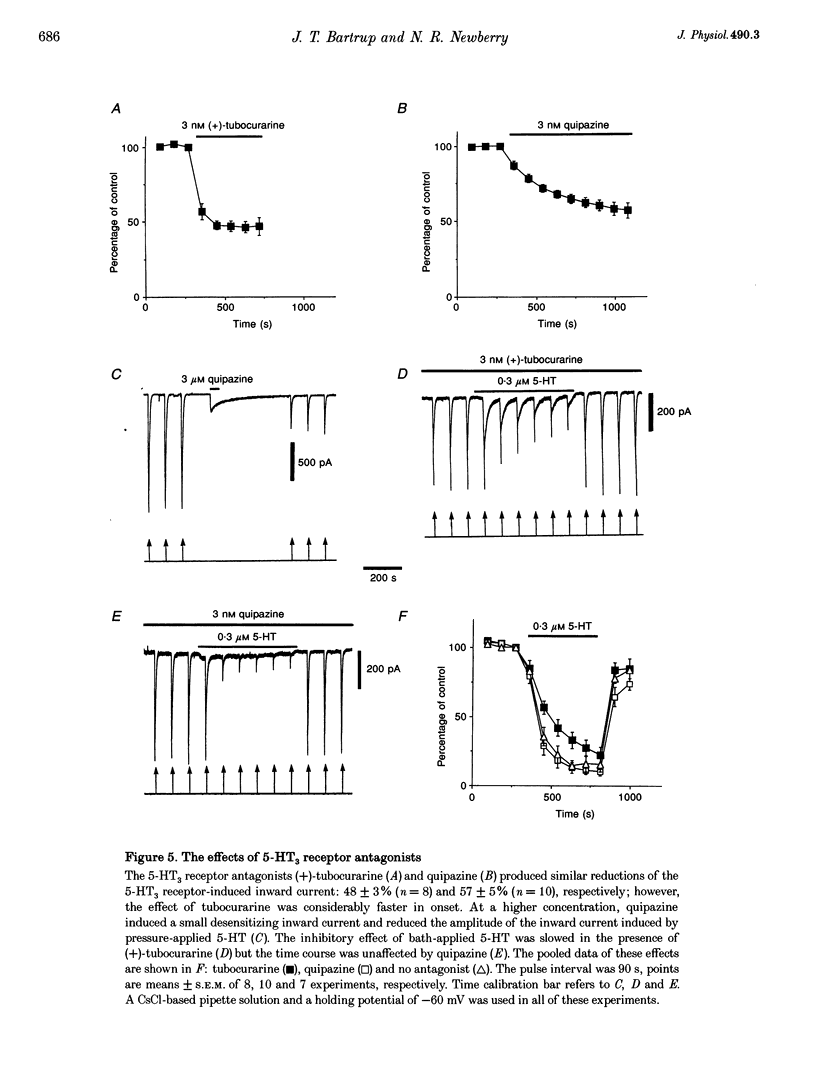
1. Using the whole-cell variation of the patch-clamp technique to record from mammalian NG108-15 cells, we have studied the ligand-gated ion channel current activated by a high concentration (100 microM) of local pressure-applied 5-hydroxytryptamine (5-HT). The response was induced at intervals of at least 90-120 s, which allowed the receptor to fully recover between activations. 2. The rapid inward current induced by pressure-applied 5-HT was reproducibly inhibited by the superfusion of low concentrations of 5-HT which evoked little or no detectable inward current alone (0.01-0.3 microM). This inhibitory effect was most likely to be due to a direct action on the 5-HT3 receptor as it could be recorded using intracellular solutions with or without adenosine triphosphate (ATP) and guanosine triphosphate (GTP). 3. The maximum inhibitory effect of a given concentration of 5-HT was not dependent on its superfusion time but on the number of activations of the receptor by pressure-applied 5-HT. This activation dependence was clearly evident, since the first inward current in the presence of 0.1 microM 5-HT was often unaffected in amplitude. 4. The inhibitory effect of 5-HT was evident at holding potentials of +60 and -60 mV; with the calcium chelator BAPTA in the recording pipette and with the nominal removal of extracellular calcium and magnesium ions. 5. The inhibitory effect was concentration dependent, with 50% inhibition of the inward current amplitude occurring at approximately 50 nM 5-HT. The slope factor of the inhibition curve was 1.3. The effect was mimicked by two other 5-HT3 receptor agonists, 2-methyl-5-HT and m-chlorophenylbiguanide (mCPBG) which gave 50% inhibition at approximately 600 nM and approximately 20 nM, respectively. These values are similar to the affinity values for these ligands determined in radioligand binding assays. 6. The 5-HT3 receptor "antagonists' (+)-tubocurarine and quipazine (both at 3 nM) reduced the inward current amplitude by approximately 50%. The rate of onset of the inhibitory effect of bath-applied 5-HT was slowed in the presence of (+)-tubocurarine but not in the presence of quipazine. This difference might be explained by the agonist properties seen only with quipazine. 7. The inhibition of the 5-HT3 receptor mediated inward current by low concentrations of bath-applied 5-HT3 receptor agonists is compatible with the cyclic model of receptor activation and desensitization. We conclude that we have been studying the high-affinity binding of agonists to the desensitized form of the 5-HT3 receptor.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. M., Barnes N. M. Differential binding characteristics of agonists at 5-HT3 receptor recognition sites in NG108-15 neuroblastoma-glioma cells labelled by [3H]-(S)-zacopride and [3H]granisetron. Biochem Pharmacol. 1993 May 25;45(10):2155–2158. doi: 10.1016/0006-2952(93)90030-z. [DOI] [PubMed] [Google Scholar]
- Boess F. G., Beroukhim R., Martin I. L. Ultrastructure of the 5-hydroxytryptamine3 receptor. J Neurochem. 1995 Mar;64(3):1401–1405. doi: 10.1046/j.1471-4159.1995.64031401.x. [DOI] [PubMed] [Google Scholar]
- Boess F. G., Sepúlveda M. I., Lummis S. C., Martin I. L. 5-HT3 receptors in NG108-15 neuroblastoma x glioma cells: effect of the novel agonist 1-(m-chlorophenyl)-biguanide. Neuropharmacology. 1992 Jun;31(6):561–564. doi: 10.1016/0028-3908(92)90188-u. [DOI] [PubMed] [Google Scholar]
- Derkach V., Surprenant A., North R. A. 5-HT3 receptors are membrane ion channels. Nature. 1989 Jun 29;339(6227):706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Downie D. L., Hope A. G., Belelli D., Lambert J. J., Peters J. A., Bentley K. R., Steward L. J., Chen C. Y., Barnes N. M. The interaction of trichloroethanol with murine recombinant 5-HT3 receptors. Br J Pharmacol. 1995 Apr;114(8):1641–1651. doi: 10.1111/j.1476-5381.1995.tb14952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiselé J. L., Bertrand S., Galzi J. L., Devillers-Thiéry A., Changeux J. P., Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993 Dec 2;366(6454):479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Emerit M. B., Riad M., Fattaccini C. M., Hamon M. Characteristics of [14C]guanidinium accumulation in NG 108-15 cells exposed to serotonin 5-HT3 receptor ligands and substance P. J Neurochem. 1993 Jun;60(6):2059–2067. doi: 10.1111/j.1471-4159.1993.tb03490.x. [DOI] [PubMed] [Google Scholar]
- Fan P. Effects of antidepressants on the inward current mediated by 5-HT3 receptors in rat nodose ganglion neurones. Br J Pharmacol. 1994 Jul;112(3):741–744. doi: 10.1111/j.1476-5381.1994.tb13140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope A. G., Downie D. L., Sutherland L., Lambert J. J., Peters J. A., Burchell B. Cloning and functional expression of an apparent splice variant of the murine 5-HT3 receptor A subunit. Eur J Pharmacol. 1993 Apr 15;245(2):187–192. doi: 10.1016/0922-4106(93)90128-v. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick G. J., Jones B. J., Tyers M. B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987 Dec 24;330(6150):746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Kooyman A. R., Zwart R., Vijverberg H. P. Tetraethylammonium ions block 5-HT3 receptor-mediated ion current at the agonist recognition site and prevent desensitization in cultured mouse neuroblastoma cells. Eur J Pharmacol. 1993 Aug 15;246(3):247–254. doi: 10.1016/0922-4106(93)90038-b. [DOI] [PubMed] [Google Scholar]
- Kooyman A. R., van Hooft J. A., Vijverberg H. P. 5-Hydroxyindole slows desensitization of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells. Br J Pharmacol. 1993 Feb;108(2):287–289. doi: 10.1111/j.1476-5381.1993.tb12795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Stevens C. F. Both open and closed NMDA receptor channels desensitize. J Neurosci. 1994 Apr;14(4):2153–2160. doi: 10.1523/JNEUROSCI.14-04-02153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq A. V., Peterson A. S., Brake A. J., Myers R. M., Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991 Oct 18;254(5030):432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Neijt H. C., Plomp J. J., Vijverberg H. P. Kinetics of the membrane current mediated by serotonin 5-HT3 receptors in cultured mouse neuroblastoma cells. J Physiol. 1989 Apr;411:257–269. doi: 10.1113/jphysiol.1989.sp017572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijt H. C., te Duits I. J., Vijverberg H. P. Pharmacological characterization of serotonin 5-HT3 receptor-mediated electrical response in cultured mouse neuroblastoma cells. Neuropharmacology. 1988 Mar;27(3):301–307. doi: 10.1016/0028-3908(88)90048-2. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Cheshire S. H., Gilbert M. J. Evidence that the 5-HT3 receptors of the rat, mouse and guinea-pig superior cervical ganglion may be different. Br J Pharmacol. 1991 Mar;102(3):615–620. doi: 10.1111/j.1476-5381.1991.tb12221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Watkins C. J., Sprosen T. S., Blackburn T. P., Grahame-Smith D. G., Leslie R. A. BRL 46470 potently antagonizes neural responses activated by 5-HT3 receptors. Neuropharmacology. 1993 Aug;32(8):729–735. doi: 10.1016/0028-3908(93)90180-b. [DOI] [PubMed] [Google Scholar]
- Ortells M. O., Lunt G. G. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995 Mar;18(3):121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Hales T. G., Lambert J. J. Divalent cations modulate 5-HT3 receptor-induced currents in N1E-115 neuroblastoma cells. Eur J Pharmacol. 1988 Jul 14;151(3):491–495. doi: 10.1016/0014-2999(88)90550-x. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Malone H. M., Lambert J. J. An electrophysiological investigation of the properties of 5-HT3 receptors of rabbit nodose ganglion neurones in culture. Br J Pharmacol. 1993 Oct;110(2):665–676. doi: 10.1111/j.1476-5381.1993.tb13863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. A., Malone H. M., Lambert J. J. Antagonism of 5-HT3 receptor mediated currents in murine N1E-115 neuroblastoma cells by (+)-tubocurarine. Neurosci Lett. 1990 Mar 2;110(1-2):107–112. doi: 10.1016/0304-3940(90)90796-c. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Malone H. M., Lambert J. J. Recent advances in the electrophysiological characterization of 5-HT3 receptors. Trends Pharmacol Sci. 1992 Oct;13(10):391–397. doi: 10.1016/0165-6147(92)90119-q. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Robertson B., Bevan S. Properties of 5-hydroxytryptamine3 receptor-gated currents in adult rat dorsal root ganglion neurones. Br J Pharmacol. 1991 Jan;102(1):272–276. doi: 10.1111/j.1476-5381.1991.tb12165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropert N., Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol. 1991 Sep;441:121–136. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda M. I., Lummis S. C., Martin I. L. The agonist properties of m-chlorophenylbiguanide and 2-methyl-5-hydroxytryptamine on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol. 1991 Oct;104(2):536–540. doi: 10.1111/j.1476-5381.1991.tb12464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Shen K. Z., North R. A. 5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992 Jan;8(1):199–203. doi: 10.1016/0896-6273(92)90121-s. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr Calcium-mediated modulation of N-methyl-D-aspartate (NMDA) responses in cultured rat hippocampal neurones. J Physiol. 1993 Oct;470:575–600. doi: 10.1113/jphysiol.1993.sp019876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Lagrutta A., Adelman J. P., North R. A. Single amino acid substitution affects desensitization of the 5-hydroxytryptamine type 3 receptor expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5030–5033. doi: 10.1073/pnas.90.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J. L., Shao X. M., Jackson M. B. Activation and desensitization of the 5-HT3 receptor in a rat glioma x mouse neuroblastoma hybrid cell. J Physiol. 1991 May;436:293–308. doi: 10.1113/jphysiol.1991.sp018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J Gen Physiol. 1990 Dec;96(6):1177–1198. doi: 10.1085/jgp.96.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]


