Abstract
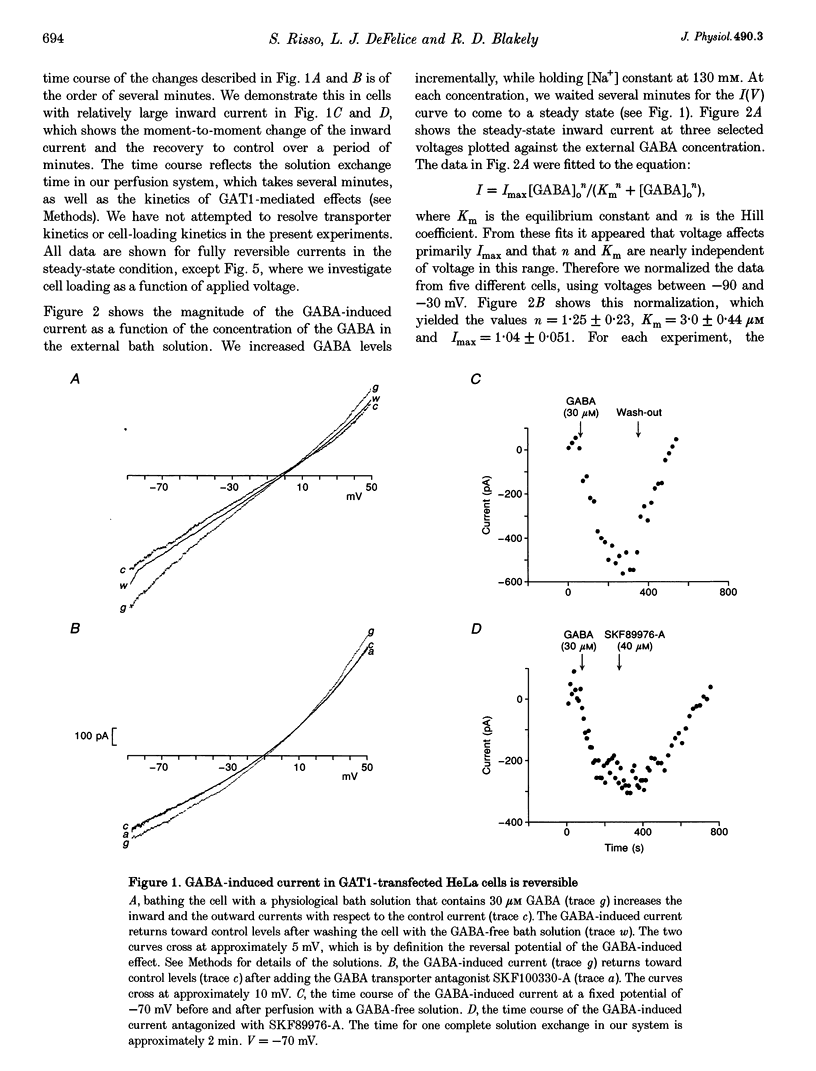
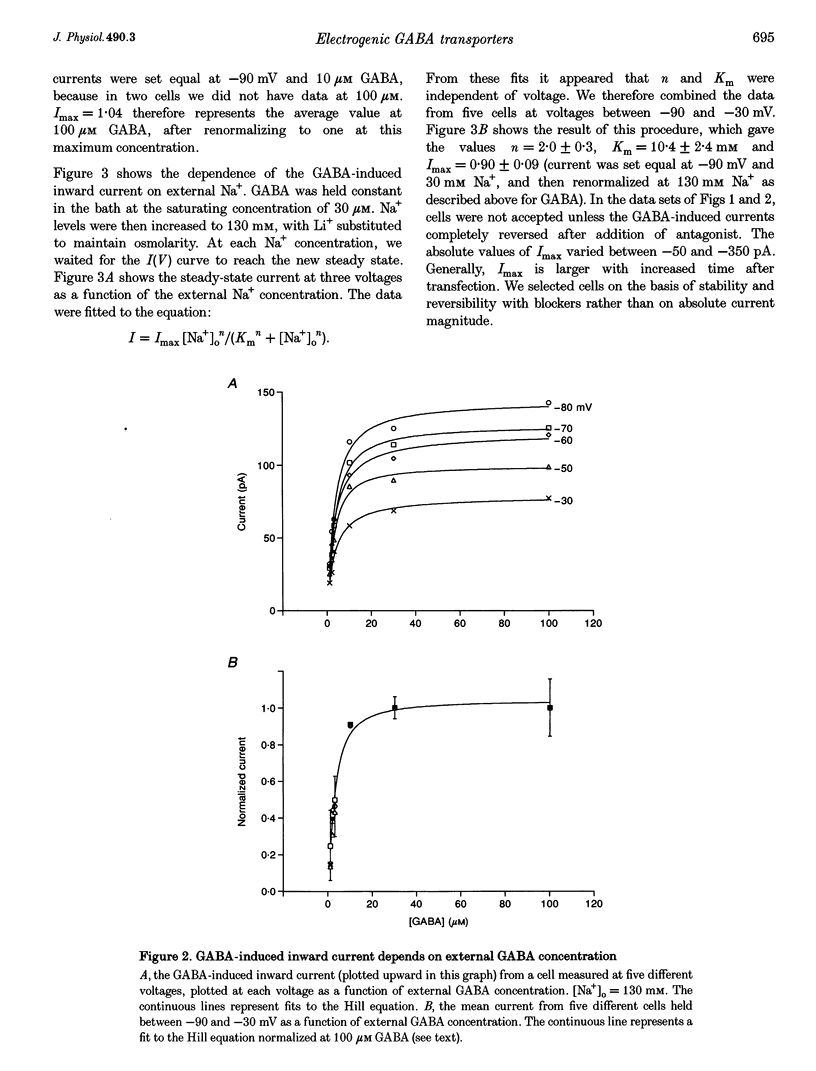
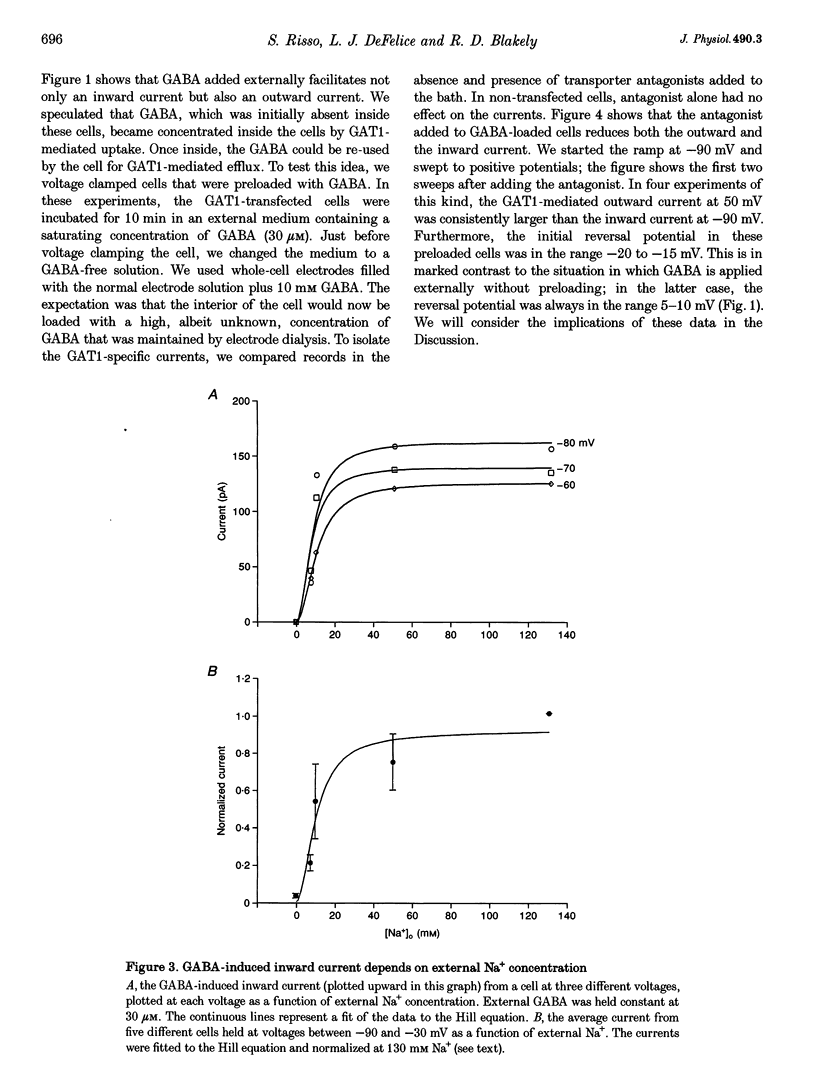
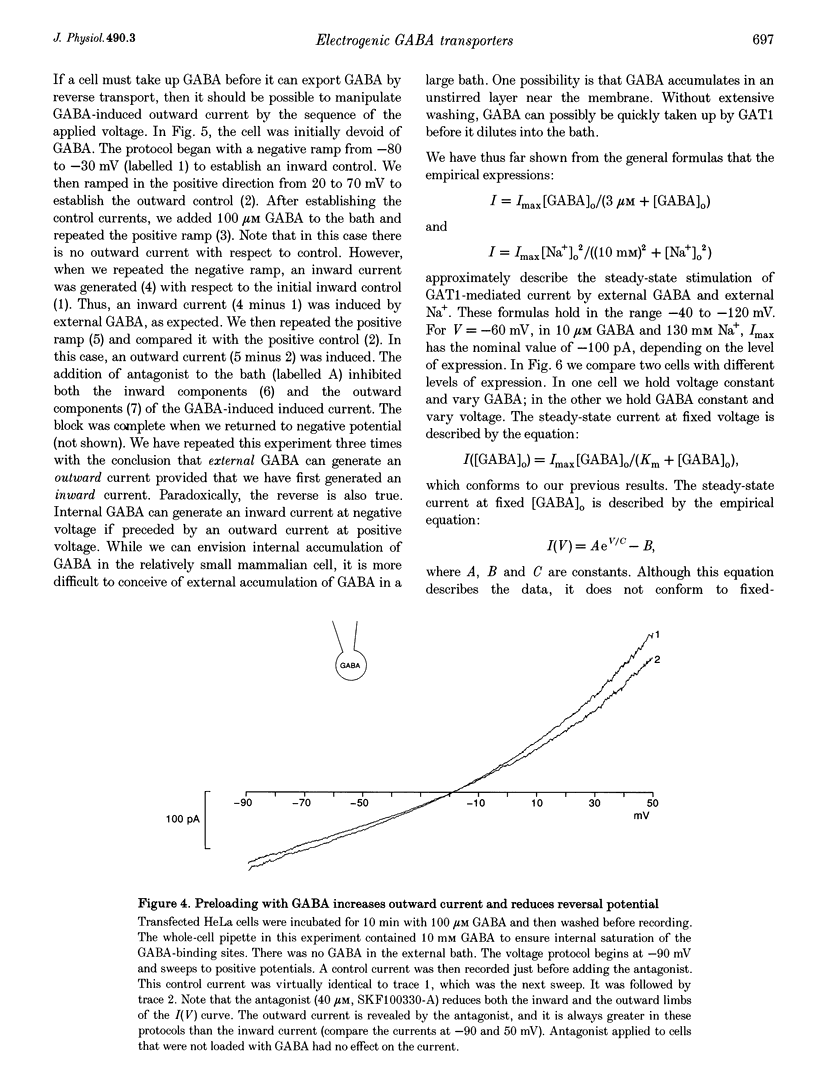
1. HeLa cells were infected with recombinant vaccinia virus containing the T7 RNA polymerase gene and transfected with the cDNA for a rat GABA transporter, GAT1, cloned downstream of a T7 RNA polymerase promoter. Six to sixteen hours after transfection, whole-cell recording with a voltage ramp in the range -90 to 50 mV revealed GABA-induced currents (approximately -100 pA at -60 mV in 100 microM GABA, 16 h after transfection at room temperature). No GABA-induced currents were observed in parental HeLa cells or in mock-transfected cells. 2. GABA-induced currents were suppressed by extracellular perfusion with GABA-free solutions or addition of GAT1 inhibitors SKF89976-A or SKF100330-A. At fixed voltage the GABA dependence of the inward current fitted the Michaelis-Menten equation with a Hill coefficient, n, near unity and an equilibrium constant, K(m), near 3 microM. The Na+ dependence of the inward currents fitted the Michaelis-Menten equation with n approximately equal to 2 and K(m) approximately equal to 10 mM. The constants n and K(m) for GABA and Na+ were independent of voltage in the range -90 to -30 mV. 3. GABA-induced currents reverse direction in the range 5-10 mV. The implication of this result is that GAT1 can mediate electrogenic (electrophoretic) influx or efflux of GABA depending on the membrane voltage. The presence of an outward current in our experiments is consistent with radioactive-labelled flux data from resealed vesicle studies. However, it is inconsistent with frog oocyte expression experiments using the sample clone. In oocytes, GAT1 generates no outward current in a similar voltage range. Smaller intracellular volume or higher turnover rates in the mammalian expression system may explain the outward currents. 4. External GABA induces inward current, and internal GABA induces outward current. However, in cells initially devoid of internal GABA, external GABA can also facilitate an outward current. This GAT1-mediated outward current occurs only after applying negative potentials to the cell. These data are consistent with the concept that negative potentials drive GABA and Na+ into the cell, which then leads to electrogenic efflux through GAT1 at positive voltages. 5. Assuming coupled transport, we estimate the number of transporters, N, times the turnover rate, r, to be Nr approximately 10(9) s-1 under nominal conditions (V = -60 mV, 30 microM GABA, 130 mM Na+ and room temperature). This indicates either very high levels of expression (approximately 10(4) microns-2), assuming published turnover rates (approximately 10 s-1), or turnover rates that are significantly greater than previously reported. As an alternative, a channel may exist in the GAT1 protein that is gated by GABA and Na+ and blocked by GAT1 antagonists. The channel mode of conduction would exist in addition to the coupled, fixed-stoichiometry transporter mode of conduction.
Full text
PDF


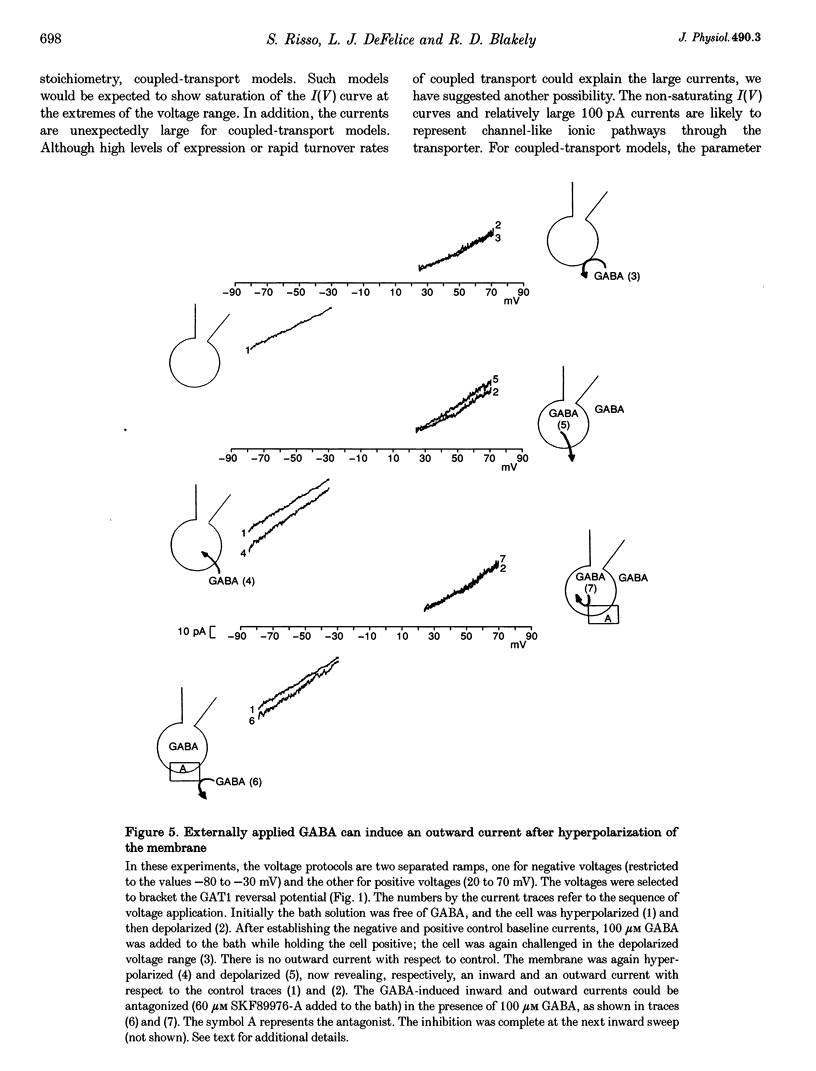
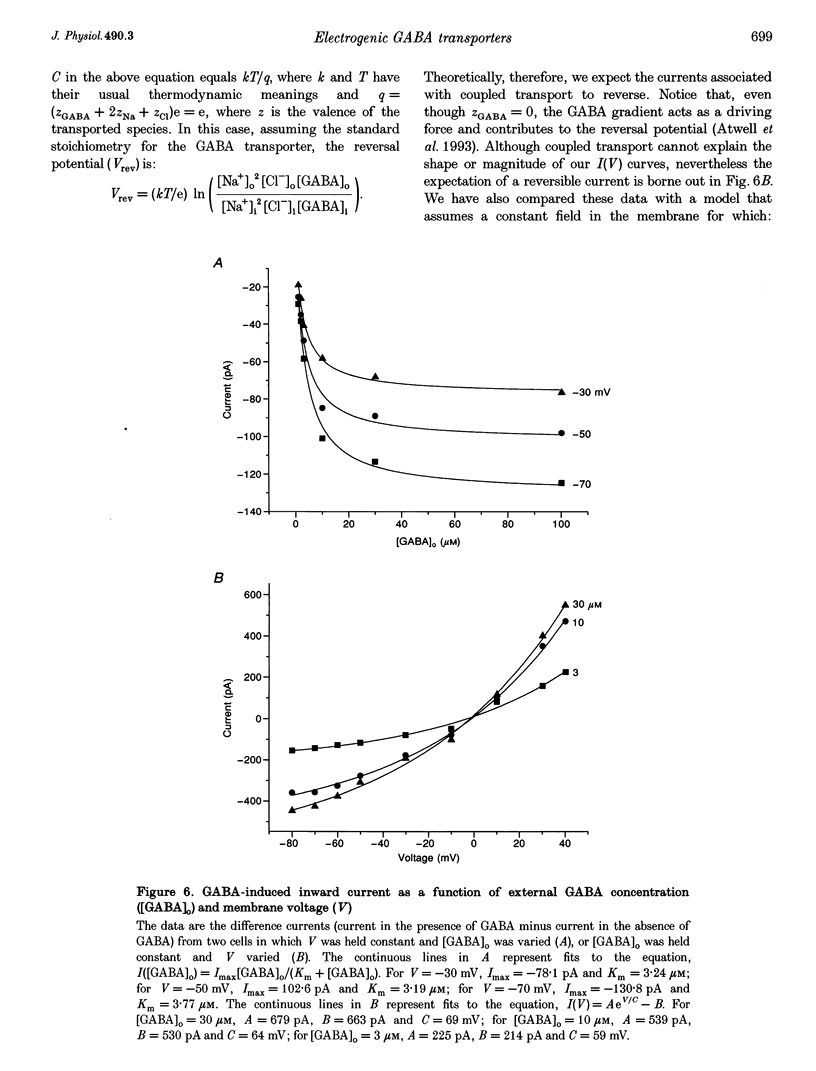



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam-Vizi V. External Ca(2+)-independent release of neurotransmitters. J Neurochem. 1992 Feb;58(2):395–405. doi: 10.1111/j.1471-4159.1992.tb09736.x. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Kuhar M. J. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Attwell D., Barbour B., Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993 Sep;11(3):401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Attwell D., Mobbs P. Neurotransmitter transporters. Curr Opin Neurobiol. 1994 Jun;4(3):353–359. doi: 10.1016/0959-4388(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Burstyn P. G., Firth D. R. The form of sodium-calcium competition at the frog myoneural junction. J Gen Physiol. 1968 Dec;52(6):887–907. doi: 10.1085/jgp.52.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely R. D., Clark J. A., Rudnick G., Amara S. G. Vaccinia-T7 RNA polymerase expression system: evaluation for the expression cloning of plasma membrane transporters. Anal Biochem. 1991 May 1;194(2):302–308. doi: 10.1016/0003-2697(91)90233-j. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., De Felice L. J., Hartzell H. C. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994 Nov;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., King A. C. Influence of membrane potential on the sodium-dependent uptake of gamma-aminobutyric acid by presynaptic nerve terminals: experimental observations and theoretical considerations. J Membr Biol. 1976 Dec 28;30(2):153–173. doi: 10.1007/BF01869665. [DOI] [PubMed] [Google Scholar]
- Cammack J. N., Rakhilin S. V., Schwartz E. A. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994 Oct;13(4):949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Clark J. A., Deutch A. Y., Gallipoli P. Z., Amara S. G. Functional expression and CNS distribution of a beta-alanine-sensitive neuronal GABA transporter. Neuron. 1992 Aug;9(2):337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- Cunningham J. R., Neal M. J., Stone S., Witkovsky P. GABA release from Xenopus retina does not correlate with horizontal cell membrane potential. Neuroscience. 1988 Jan;24(1):39–48. doi: 10.1016/0306-4522(88)90309-0. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN A. CARRIER MODEL FOR ACTIVE TRANSPORT OF IONS ACROSS A MOSAIC MEMBRANE. Biophys J. 1964 Nov;4:421–440. doi: 10.1016/s0006-3495(64)86793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman W. A., Vandenberg R. J., Arriza J. L., Kavanaugh M. P., Amara S. G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995 Jun 15;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A., DeFelice L. J., Duke B. J., Moore K. R., Blakely R. D. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol. 1995 Oct;198(Pt 10):2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hansen U. P., Gradmann D., Sanders D., Slayman C. L. Interpretation of current-voltage relationships for "active" ion transport systems: I. Steady-state reaction-kinetic analysis of class-I mechanisms. J Membr Biol. 1981;63(3):165–190. doi: 10.1007/BF01870979. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Miller R. J. Excitatory amino acid-evoked release of [3H]GABA from hippocampal neurons in primary culture. Brain Res. 1989 Mar 13;482(1):23–33. doi: 10.1016/0006-8993(89)90538-6. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Bendahan A., Radian R. Efflux and exchange of gamma-aminobutyric acid and nipecotic acid catalysed by synaptic plasma membrane vesicles isolated from immature rat brain. Biochim Biophys Acta. 1983 May 26;731(1):54–62. doi: 10.1016/0005-2736(83)90397-8. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Arriza J. L., North R. A., Amara S. G. Electrogenic uptake of gamma-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J Biol Chem. 1992 Nov 5;267(31):22007–22009. [PubMed] [Google Scholar]
- Keynan S., Kanner B. I. gamma-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry. 1988 Jan 12;27(1):12–17. doi: 10.1021/bi00401a003. [DOI] [PubMed] [Google Scholar]
- Keynan S., Suh Y. J., Kanner B. I., Rudnick G. Expression of a cloned gamma-aminobutyric acid transporter in mammalian cells. Biochemistry. 1992 Feb 25;31(7):1974–1979. doi: 10.1021/bi00122a011. [DOI] [PubMed] [Google Scholar]
- Liu Q. R., López-Corcuera B., Mandiyan S., Nelson H., Nelson N. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected]. J Biol Chem. 1993 Jan 25;268(3):2106–2112. [PubMed] [Google Scholar]
- Mager S., Naeve J., Quick M., Labarca C., Davidson N., Lester H. A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993 Feb;10(2):177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Malchow R. P., Ripps H. Effects of gamma-aminobutyric acid on skate retinal horizontal cells: evidence for an electrogenic uptake mechanism. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8945–8949. doi: 10.1073/pnas.87.22.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. L. Kinetics of the sodium-dependent transport of gamma-aminobutyric acid by synaptosomes. J Neurochem. 1973 Aug;21(2):345–356. doi: 10.1111/j.1471-4159.1973.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Blaustein M. P. GABA efflux from synaptosomes: effects of membrane potential, and external GABA and cations. J Membr Biol. 1982;69(3):213–223. doi: 10.1007/BF01870400. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Radian R., Kanner B. I. Stoichiometry of sodium- and chloride-coupled gamma-aminobutyric acid transport by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry. 1983 Mar 1;22(5):1236–1241. doi: 10.1021/bi00274a038. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987 Oct 16;238(4825):350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A., Tachibana M. Electrophysiology of glutamate and sodium co-transport in a glial cell of the salamander retina. J Physiol. 1990 Jul;426:43–80. doi: 10.1113/jphysiol.1990.sp018126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinyard E. A., White H. S., Wolf H. H., Bondinell W. E. Anticonvulsant profiles of the potent and orally active GABA uptake inhibitors SK&F 89976-A and SK&F 100330-A and four prototype antiepileptic drugs in mice and rats. Epilepsia. 1991 Jul-Aug;32(4):569–577. doi: 10.1111/j.1528-1157.1991.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol. 1992 Jun;67(6):1698–1701. doi: 10.1152/jn.1992.67.6.1698. [DOI] [PubMed] [Google Scholar]
- Umbach J. A., Coady M. J., Wright E. M. Intestinal Na+/glucose cotransporter expressed in Xenopus oocytes is electrogenic. Biophys J. 1990 Jun;57(6):1217–1224. doi: 10.1016/S0006-3495(90)82640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. Excitatory amino acid-evoked release of gamma-[3H]aminobutyric acid from striatal neurons in primary culture. J Neurochem. 1988 Aug;51(2):435–441. doi: 10.1111/j.1471-4159.1988.tb01057.x. [DOI] [PubMed] [Google Scholar]


