Abstract
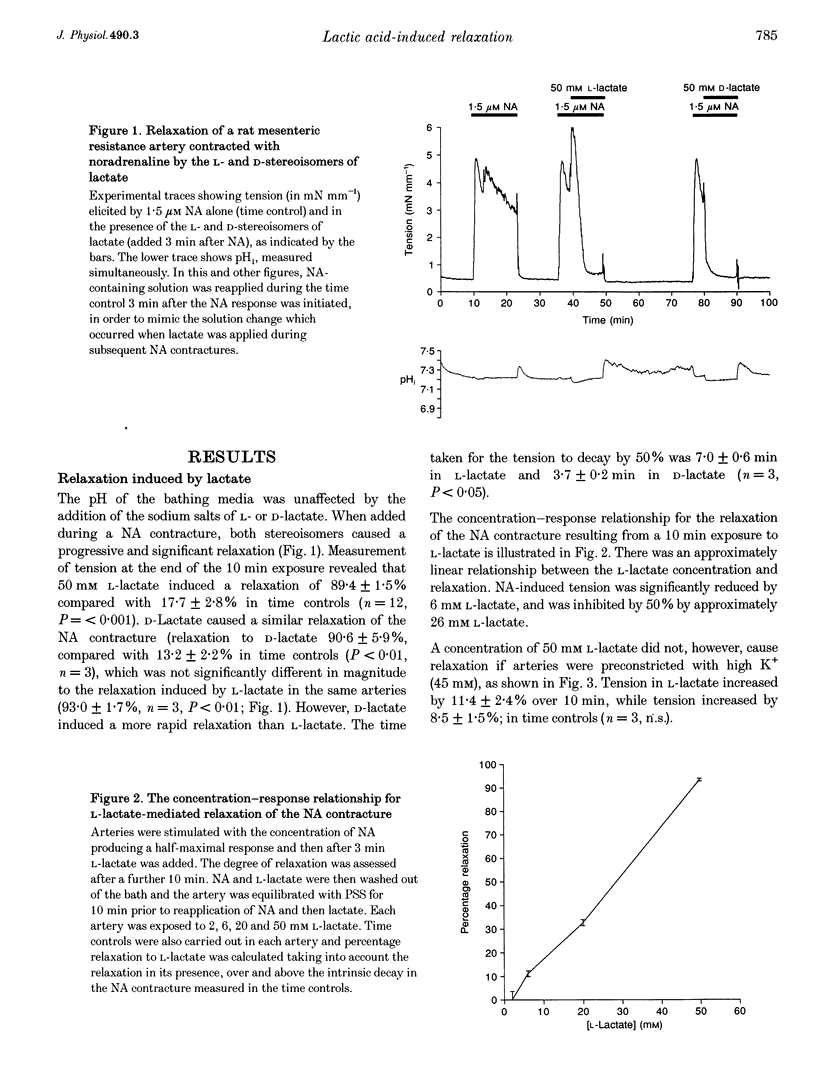
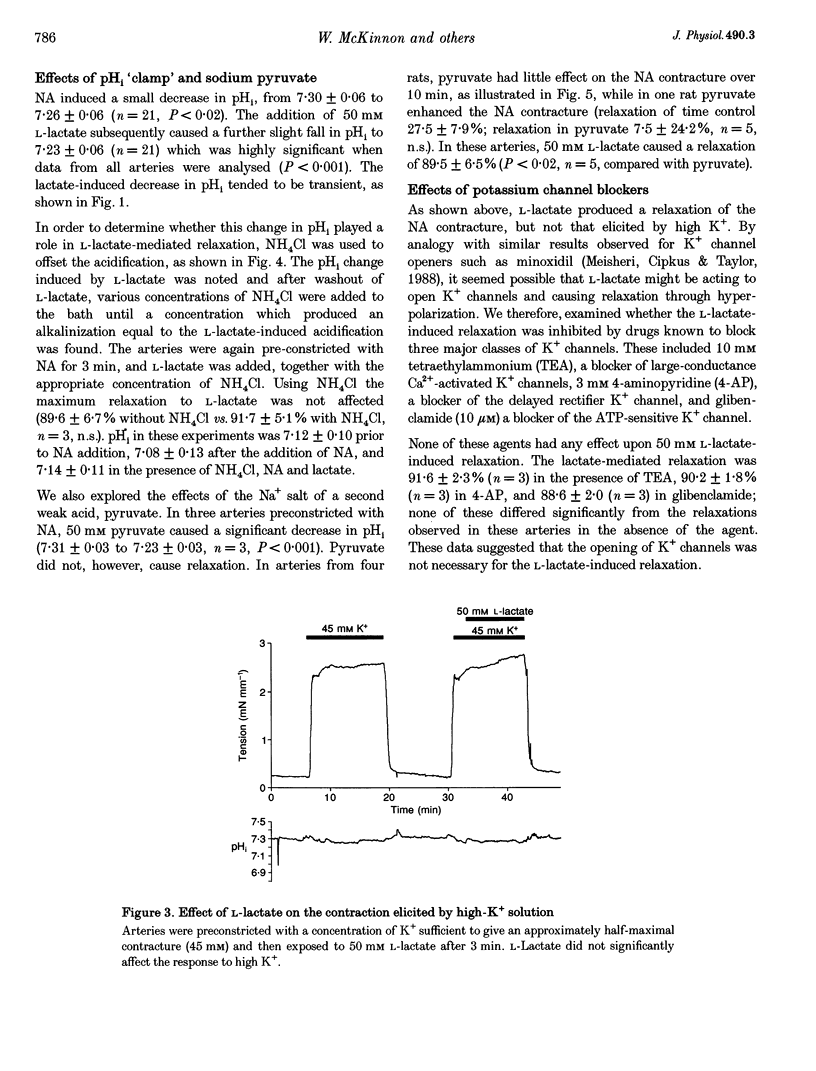
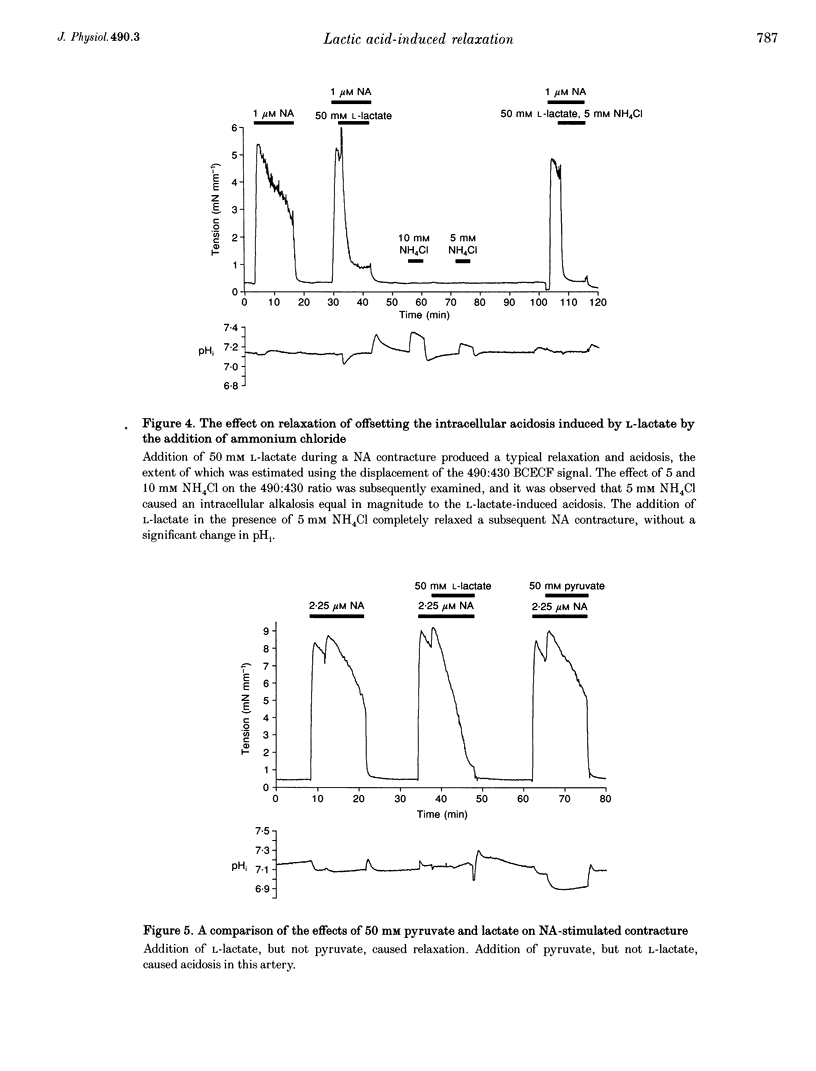
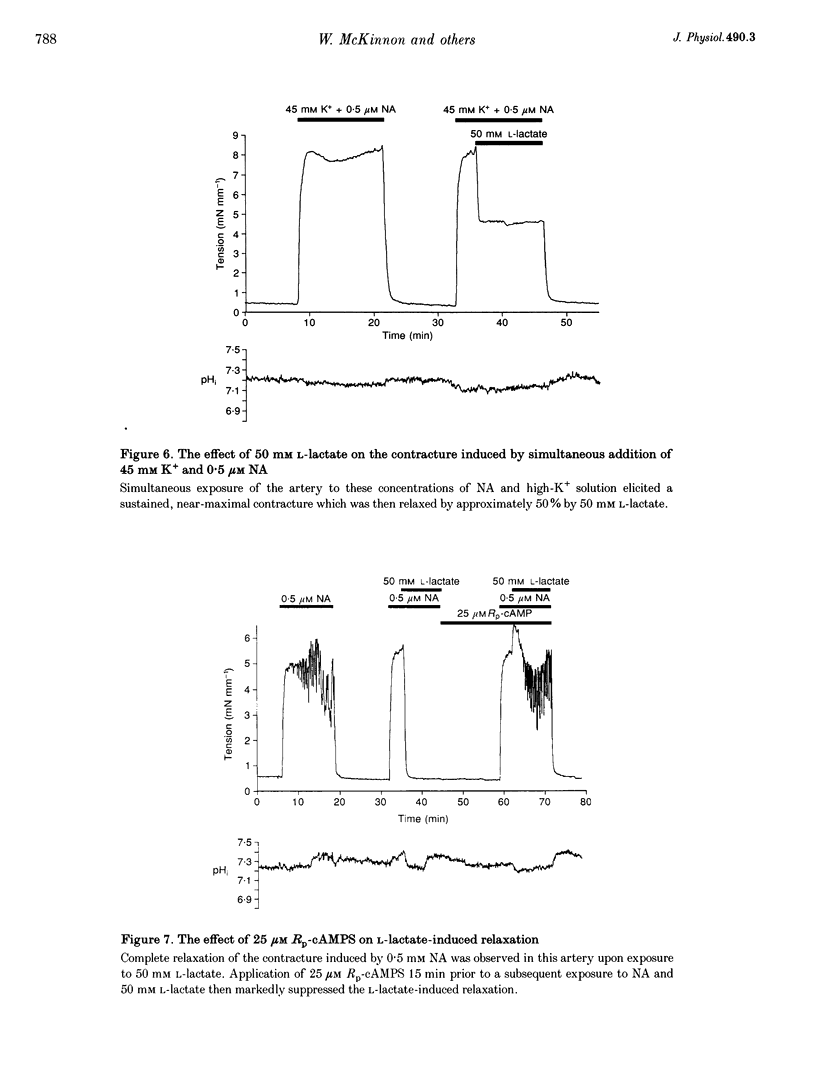
1. The effects of the sodium salt of the weak acid lactate on tension and intracellular pH (pH1) were studied in rat mesenteric small arteries mounted on a wire myograph. Sodium lactate was substituted iso-osmotically for sodium chloride. 2. At a concentration of 50 mM, both L- and D-stereoisomers of lactate markedly relaxed arteries preconstricted with noradrenaline (NA) within 10 min. The concentration-response relationship for L-lactate showed that the NA contracture was relaxed by 50% at approximately 26 mM. L-Lactate did not, however, relax arteries preconstricted with high-K+(45 mM) solution. 3. L-Lactate did not alter extracellular pH (pHo) but caused a small but significant decrease in pH1, measured using the pH-sensitive fluorochrome, 2',7'-bis(carboxyethyl)-5-(6)-carboxyfluorescein (BCECF). Relaxation to L-lactate was unaffected when this change in pHi was offset by the simultaneous addition of NH4Cl to the solution. 4. Sodium pyruvate (50 mM) caused a significant intracellular acidosis but did not relax arteries preconstricted with NA. 5. L-Lactate-induced relaxations were unaffected by removal of the endothelium or when the synthesis of nitric oxide (NO) was inhibited by 10(-4) M N omega-nitro-L-arginine methyl ester (L-NAME). 6. The potassium channel blockers glibenclamide (10 microM), 4-aminopyridine (3 mM) and tetraethylammonium chloride (10 mM) did not affect L-lactate-induced relaxation in arteries preconstricted with NA. Inhibition of guanylate cyclase with Methylene Blue, or cyclooxgenase with indomethacin, also did not affect relaxation to L-lactate. 7. The Rp stereoisomer of adenosine-3',5'-cyclic monophosphothioate (Rp-cAMPS), an analogue of cAMP which inhibits competitively stimulation of protein kinase A, reduced significantly L-lactate-induced relaxation at a concentration of 25 microM. Rp-cAMPS also significantly reduced forskolin-induced relaxation of the NA contracture. 8. It is concluded that L-lactate-induced relaxation in this vascular bed is pHi-1 endothelium-, and nitric oxide-independent. It is not mediated by inhibition of voltage-gated Ca2+ channels, opening of K+ channels, prostacylin or cyclic GMP. cAMP may however play a role in L-lactate-induced relaxation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Cragoe E. J., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol. 1988 Aug;402:391–410. doi: 10.1113/jphysiol.1988.sp017211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalkjaer C., Hughes A. Chloride and bicarbonate transport in rat resistance arteries. J Physiol. 1991 May;436:57–73. doi: 10.1113/jphysiol.1991.sp018539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalkjaer C., Mulvany M. J. Effect of changes in intracellular pH on the contractility of rat resistance vessels. Prog Biochem Pharmacol. 1988;23:150–158. [PubMed] [Google Scholar]
- Austin C., Wray S. A quantitative study of the relation between intracellular pH and force in rat mesenteric vascular smooth muscle. Pflugers Arch. 1994 Jun;427(3-4):270–276. doi: 10.1007/BF00374534. [DOI] [PubMed] [Google Scholar]
- Austin C., Wray S. Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol. 1993 Jul;466:1–8. [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Johansen L., Graham T., Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol. 1993 Mar;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D. C., Peces R., LaPointe M. S., Ye M., Daugirdas J. T. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol. 1993 Apr;264(4 Pt 1):C932–C943. doi: 10.1152/ajpcell.1993.264.4.C932. [DOI] [PubMed] [Google Scholar]
- Carr P., Graves J. E., Poston L. Carbon dioxide induced vasorelaxation in rat mesenteric small arteries precontracted with noradrenaline is endothelium dependent and mediated by nitric oxide. Pflugers Arch. 1993 May;423(3-4):343–345. doi: 10.1007/BF00374415. [DOI] [PubMed] [Google Scholar]
- Crichton C. A., Templeton A. G., Smith G. L. Effect of altered bathing pH on calcium activated force in alpha toxin permeabilised rat portal vein and human umbilical artery. Cardiovasc Res. 1994 Sep;28(9):1378–1384. doi: 10.1093/cvr/28.9.1378. [DOI] [PubMed] [Google Scholar]
- Daugirdas J. T., Swanson V., Islam S., Nutting C., Kim D. D., Wang X. A., Fiscus R. R. Acetate causes endothelium-independent increases in cyclic AMP in rat caudal artery. Am J Physiol. 1988 Dec;255(6 Pt 2):H1378–H1383. doi: 10.1152/ajpheart.1988.255.6.H1378. [DOI] [PubMed] [Google Scholar]
- FROHLICH E. D. VASCULAR EFFECTS OF THE KREBS INTERMEDIATE METABOLITES. Am J Physiol. 1965 Jan;208:149–153. doi: 10.1152/ajplegacy.1965.208.1.149. [DOI] [PubMed] [Google Scholar]
- HUCKABEE W. E. Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients. Am J Med. 1961 Jun;30:840–848. doi: 10.1016/0002-9343(61)90172-3. [DOI] [PubMed] [Google Scholar]
- Hilton R., Eichholtz F. The influence of chemical factors on the coronary circulation. J Physiol. 1925 Mar 31;59(6):413–425. doi: 10.1113/jphysiol.1925.sp002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E., Hughes A., Boonen H. C., Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res. 1993 Aug;73(2):314–324. doi: 10.1161/01.res.73.2.314. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. J Gen Physiol. 1994 Apr;103(4):647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takagi K., Satake T., Tokuno H., Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J Physiol. 1990 May;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Kutchai H., Geddis L. M., Martin M. S. Mediated transport and metabolism of lactate in rat aorta. Biochim Biophys Acta. 1978 Jul 3;541(3):312–320. doi: 10.1016/0304-4165(78)90191-5. [DOI] [PubMed] [Google Scholar]
- MOHME-LUNDHOLM E. Mechanism of the relaxing effect of adrenaline on bovine coronary vessels. Acta Physiol Scand. 1957 Mar 7;38(3-4):255–264. doi: 10.1111/j.1748-1716.1957.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Matthews J. G., Graves J. E., Poston L. Relationships between pHi and tension in isolated rat mesenteric resistance arteries. J Vasc Res. 1992 Jul-Aug;29(4):330–340. doi: 10.1159/000158948. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Cipkus L. A., Taylor C. J. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988 Jun;245(3):751–760. [PubMed] [Google Scholar]
- Miller F. N., Nolph K. D., Joshua I. G., Wiegman D. L., Harris P. D., Andersen D. B. Hyperosmolality, acetate, and lactate: dilatory factors during peritoneal dialysis. Kidney Int. 1981 Sep;20(3):397–402. doi: 10.1038/ki.1981.152. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Nutting C. W., Islam S., Daugirdas J. T. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol. 1991 Aug;261(2 Pt 2):H561–H567. doi: 10.1152/ajpheart.1991.261.2.H561. [DOI] [PubMed] [Google Scholar]
- Nutting C. W., Islam S., Ye M. H., Batlle D. C., Daugirdas J. T. The vasorelaxant effects of acetate: role of adenosine, glycolysis, lyotropism, and pHi and Cai2+. Kidney Int. 1992 Jan;41(1):166–174. doi: 10.1038/ki.1992.23. [DOI] [PubMed] [Google Scholar]
- Omar H. A., Figueroa R., Tejani N., Wolin M. S. Properties of a lactate-induced relaxation in human placental arteries and veins. Am J Obstet Gynecol. 1993 Oct;169(4):912–918. doi: 10.1016/0002-9378(93)90026-f. [DOI] [PubMed] [Google Scholar]
- Omar H. A., Mohazzab K. M., Mortelliti M. P., Wolin M. S. O2-dependent modulation of calf pulmonary artery tone by lactate: potential role of H2O2 and cGMP. Am J Physiol. 1993 Feb;264(2 Pt 1):L141–L145. doi: 10.1152/ajplung.1993.264.2.L141. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Kanaide H., Nakamura M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am J Physiol. 1988 Oct;255(4 Pt 2):H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Tada M., Katz A. M. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- Tashkin D. P., Goldstein P. J., Simmons D. H. Hepatic lactate uptake during decreased liver perfusion and hyposemia. Am J Physiol. 1972 Oct;223(4):968–974. doi: 10.1152/ajplegacy.1972.223.4.968. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Van Driel R., Jastorff B., Baraniak J., Stec W. J., De Wit R. J. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984 Aug 25;259(16):10020–10024. [PubMed] [Google Scholar]
- de Wit R. J., Hekstra D., Jastorff B., Stec W. J., Baraniak J., Van Driel R., Van Haastert P. J. Inhibitory action of certain cyclophosphate derivatives of cAMP on cAMP-dependent protein kinases. Eur J Biochem. 1984 Jul 16;142(2):255–260. doi: 10.1111/j.1432-1033.1984.tb08279.x. [DOI] [PubMed] [Google Scholar]


