Abstract
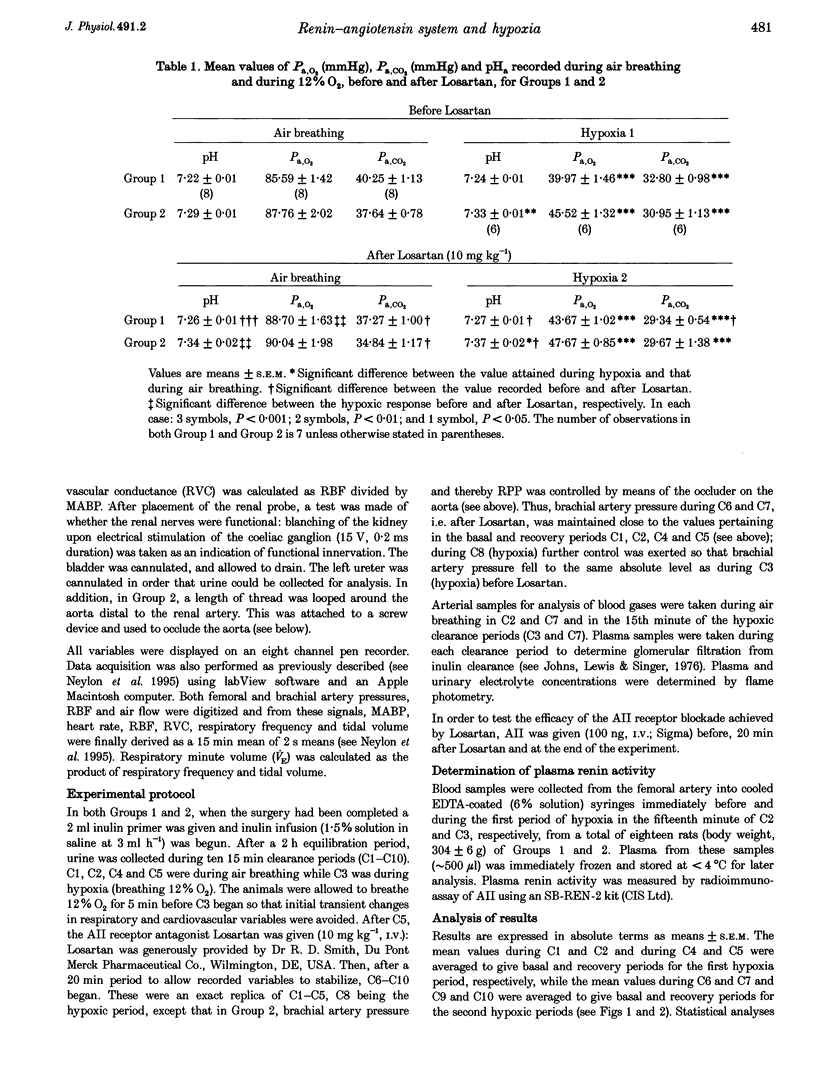
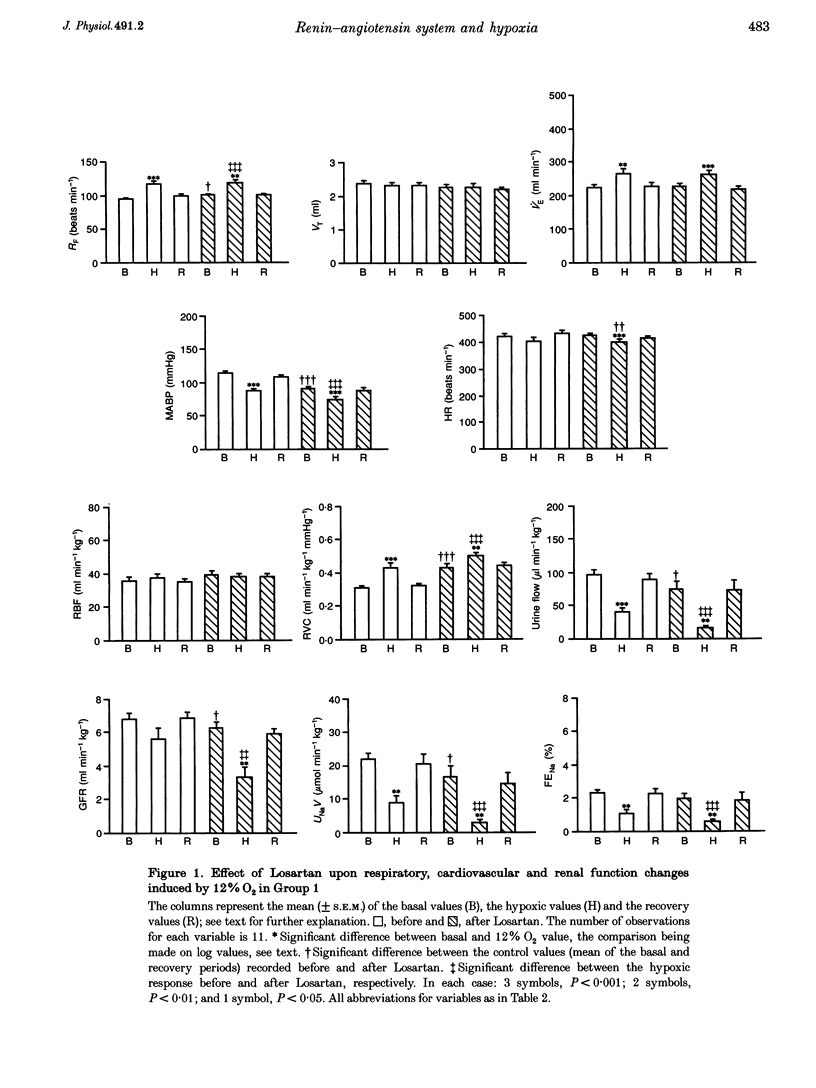
1. In two groups of Saffan-anaesthetized rats, we studied the role of the renin-angiotensin system in mediating the antidiuresis and antinatriuresis induced by moderate systemic hypoxia. 2. In both groups, a first period of hypoxia (breathing 12% O2 for 20 min) induced a fall in arterial partial pressure of O2 (Pa,O2; to 42 mmHg), a fall in mean arterial pressure (MABP), no change in renal blood flow (RBF) due to an increase in renal vascular conductance (RVC = RBF/MABP) and falls in urine flow and absolute sodium excretion (UNaV). Concomitantly, plasma renin activity increased from 3.08 +/- 0.68 (mean +/- S.E.M.) to 8.36 +/- 1.8 ng ml-1 hr-1. 3. In group 1 (n = 11), Losartan (10 mg kg-1, I.V.), the angiotensin (AII) AT1 receptor antagonist, induced a fall in MABP (115 +/- 3 to 90 +/- 3 mmHg), an increase in RVC such that RBF was unchanged, and falls in glomerular filtration rate (GFR), urine flow and UNaV. However, hypoxia induced qualitatively similar changes to those seen before Losartan treatment. 4. In group 2 (n = 9), we occluded the aorta distal to the renal artery to prevent basal MABP and renal perfusion pressure (RPP) from falling after addition of Losartan and to keep the hypoxia-induced fall in MABP the same as before Losartan treatment. Nevertheless, Losartan induced an increase in basal RVC, RBF, urine flow and UNaV whilst hypoxia induced falls in urine flow and UNaV that were proportionately similar to those seen prior to addition of Losartan. 5. These results indicate that in the Saffan-anaesthetized rat, AII exerts tonic, renal vasoconstrictor and consequent antidiuretic and antinatriuretic influences in normoxia, but does not contribute to the hypoxia-induced antidiuresis and antinatriuresis. We propose that renin secretion is increased by the hypoxia-induced fall in RPP rather than by an increase in renal sympathetic activity. Thus, the AII generated cannot produce antidiuresis and antinatriuresis by its known facilitatory influence on the actions of an increase in sympathetic activity on the renal tubules and is insufficient to produce these effects by direct actions. Rather, these results support the view that the antidiuresis and antinatriuresis of moderate hypoxia is predominantly due to the fall in RPP.
Full text
PDF


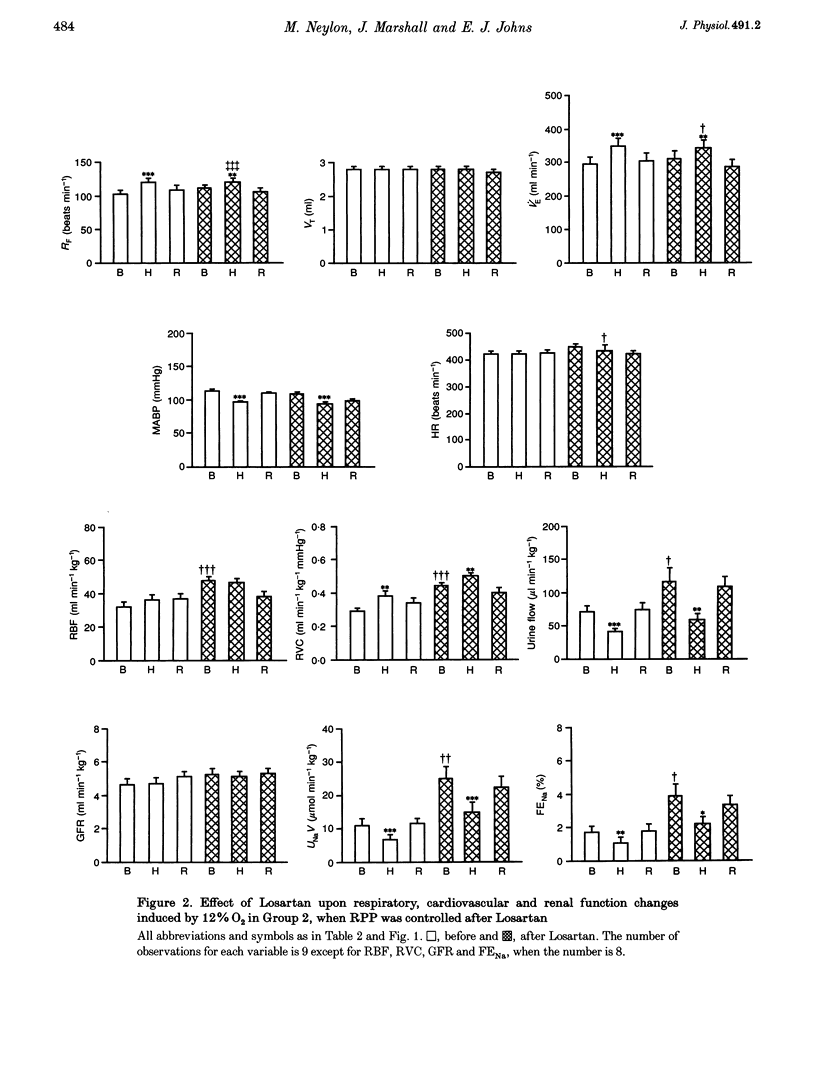




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behm R., Mewes H., DeMuinck Keizer W. H., Unger T., Rettig R. Cardiovascular and renal effects of hypoxia in conscious carotid body-denervated rats. J Appl Physiol (1985) 1993 Jun;74(6):2795–2800. doi: 10.1152/jappl.1993.74.6.2795. [DOI] [PubMed] [Google Scholar]
- Bunnemann B., Iwai N., Metzger R., Fuxe K., Inagami T., Ganten D. The distribution of angiotensin II AT1 receptor subtype mRNA in the rat brain. Neurosci Lett. 1992 Aug 17;142(2):155–158. doi: 10.1016/0304-3940(92)90362-b. [DOI] [PubMed] [Google Scholar]
- Burns K. D., Homma T., Harris R. C. The intrarenal renin-angiotensin system. Semin Nephrol. 1993 Jan;13(1):13–30. [PubMed] [Google Scholar]
- Fukuda Y., Sato A., Suzuki A., Trzebski A. Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. J Auton Nerv Syst. 1989 Oct;28(1):61–74. doi: 10.1016/0165-1838(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Handa R. K., Johns E. J. The role of angiotensin II in the renal responses to somatic nerve stimulation in the rat. J Physiol. 1987 Dec;393:425–436. doi: 10.1113/jphysiol.1987.sp016831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse I. F., Johns E. J. The role of alpha-adrenoceptors in the regulation of renal tubular sodium reabsorption and renin secretion in the rabbit. Br J Pharmacol. 1985 Mar;84(3):715–724. doi: 10.1111/j.1476-5381.1985.tb16154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes M. P., Farber M. O., Manfredi F., Robertshaw D., Weinberger M., Fineberg N., Robertson G. Acute effects of hypoxia on renal and endocrine function in normal humans. Am J Physiol. 1982 Sep;243(3):R265–R270. doi: 10.1152/ajpregu.1982.243.3.R265. [DOI] [PubMed] [Google Scholar]
- Johns E. J., Lewis B. A., Singer B. The sodium-retaining effect of renal nerve activity in the cat: role of angiotensin formation. Clin Sci Mol Med. 1976 Jul;51(1):93–102. doi: 10.1042/cs0510093. [DOI] [PubMed] [Google Scholar]
- Johns E. J. Role of angiotensin II and the sympathetic nervous system in the control of renal function. J Hypertens. 1989 Sep;7(9):695–701. [PubMed] [Google Scholar]
- Liang C. S., Gavras H. Renin-angiotensin system inhibition in conscious dogs during acute hypoxemia. Effects on systemic hemodynamics, regional blood flows, and tissue metabolism. J Clin Invest. 1978 Nov;62(5):961–970. doi: 10.1172/JCI109225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994 Jul;74(3):543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Neylon M., Marshall J. M., Johns E. J. The effects of systemic hypoxia on renal function in the anaesthetized rat. J Physiol. 1995 Sep 1;487(Pt 2):497–511. doi: 10.1113/jphysiol.1995.sp020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. L., Holdaas H., Thames M. D., DiBona G. F. Renal adrenoceptor mediation of antinatriuretic and renin secretion responses to low frequency renal nerve stimulation in the dog. Circ Res. 1983 Sep;53(3):298–305. doi: 10.1161/01.res.53.3.298. [DOI] [PubMed] [Google Scholar]
- Raff H., Roarty T. P. Renin, ACTH, and aldosterone during acute hypercapnia and hypoxia in conscious rats. Am J Physiol. 1988 Mar;254(3 Pt 2):R431–R435. doi: 10.1152/ajpregu.1988.254.3.R431. [DOI] [PubMed] [Google Scholar]
- Rose C. E., Jr, Kimmel D. P., Godine R. L., Jr, Kaiser D. L., Carey R. M. Synergistic effects of acute hypoxemia and hypercapnic acidosis in conscious dogs. Renal dysfunction and activation of the renin-angiotensin system. Circ Res. 1983 Aug;53(2):202–213. doi: 10.1161/01.res.53.2.202. [DOI] [PubMed] [Google Scholar]
- Song K. F., Zhuo J. L., Mendelsohn F. A. Access of peripherally administered DuP 753 to rat brain angiotensin II receptors. Br J Pharmacol. 1991 Dec;104(4):771–772. doi: 10.1111/j.1476-5381.1991.tb12503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F. Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci. 1991 Feb;12(2):55–62. doi: 10.1016/0165-6147(91)90498-h. [DOI] [PubMed] [Google Scholar]
- Tuffley R. E., Rubenstein D., Slater J. D., Williams E. S. Serum renin activity during exposure to hypoxia. J Endocrinol. 1970 Dec;48(4):497–510. doi: 10.1677/joe.0.0480497. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Mah S. C., Schnell C. Comparison of the acute hypotensive effects of renin inhibition, converting enzyme inhibition, and angiotensin II antagonism in rats. J Cardiovasc Pharmacol. 1990;16 (Suppl 4):S60–S64. doi: 10.1097/00005344-199016004-00013. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Whitebread S., Mele M., Motani A. S., Whitcombe P. J., Ramjoué H. P., Kamber B. Biochemical characterization of two angiotensin II receptor subtypes in the rat. J Cardiovasc Pharmacol. 1990;16 (Suppl 4):S31–S35. doi: 10.1097/00005344-199016004-00008. [DOI] [PubMed] [Google Scholar]


