Abstract
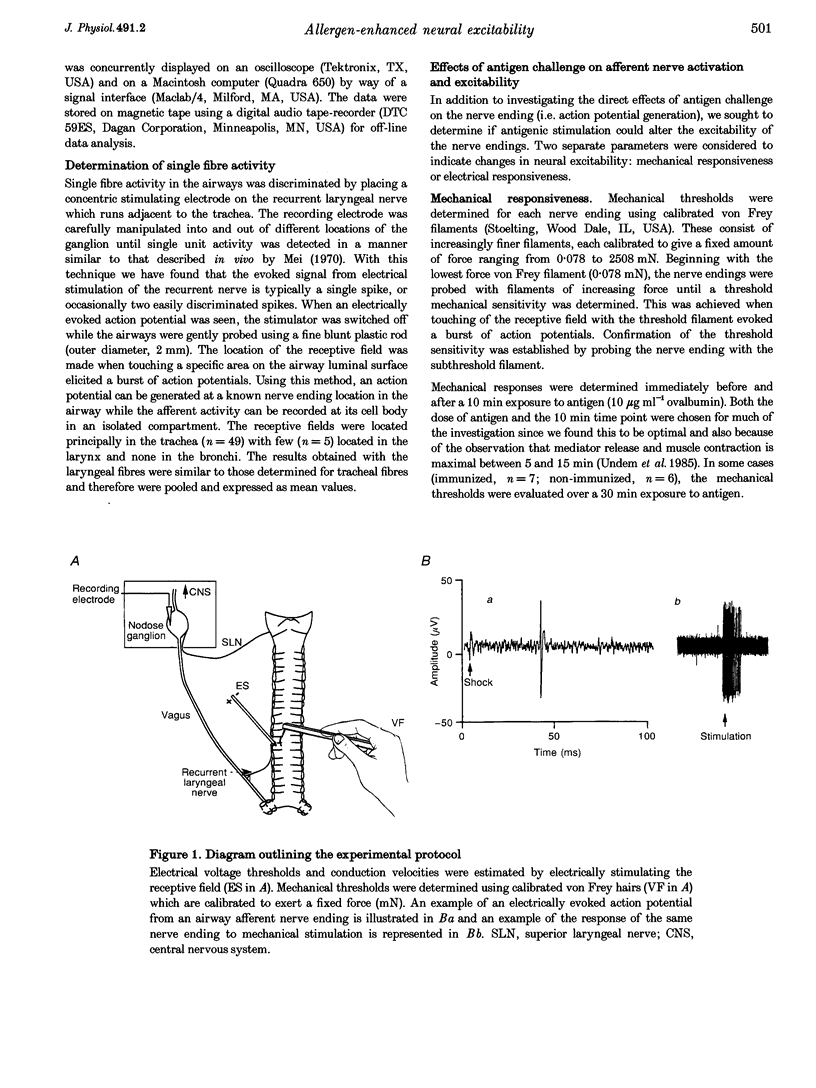
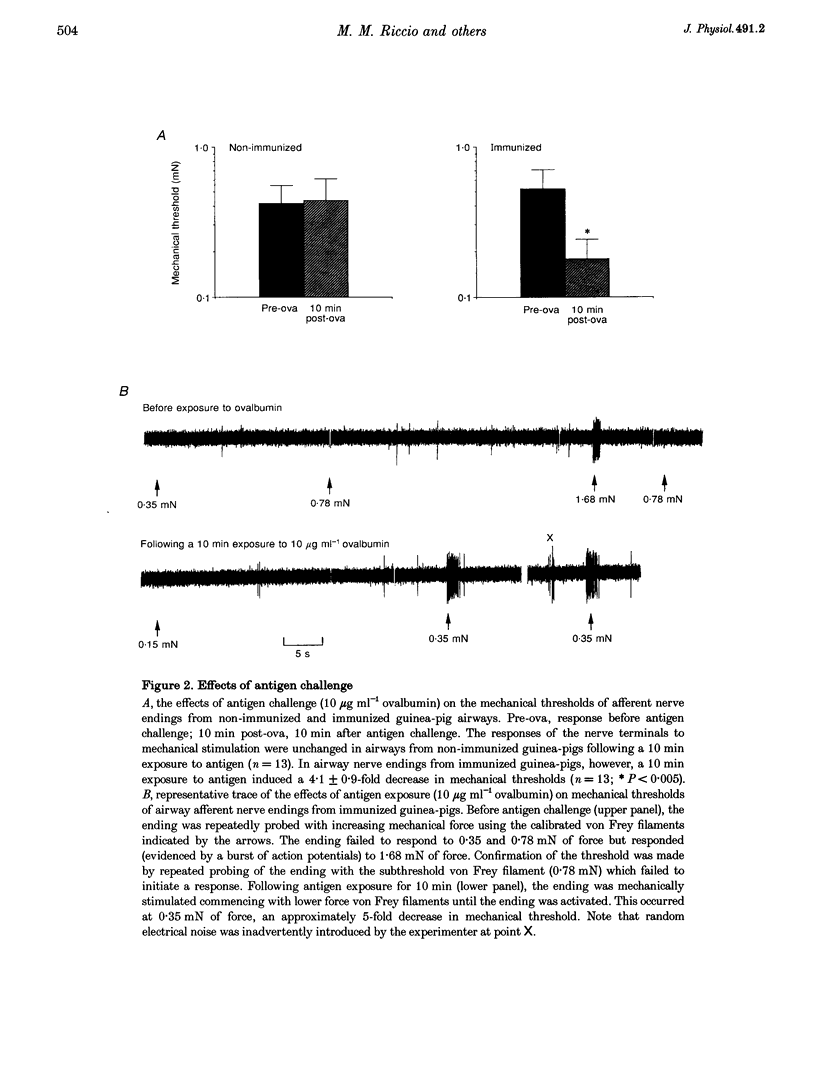
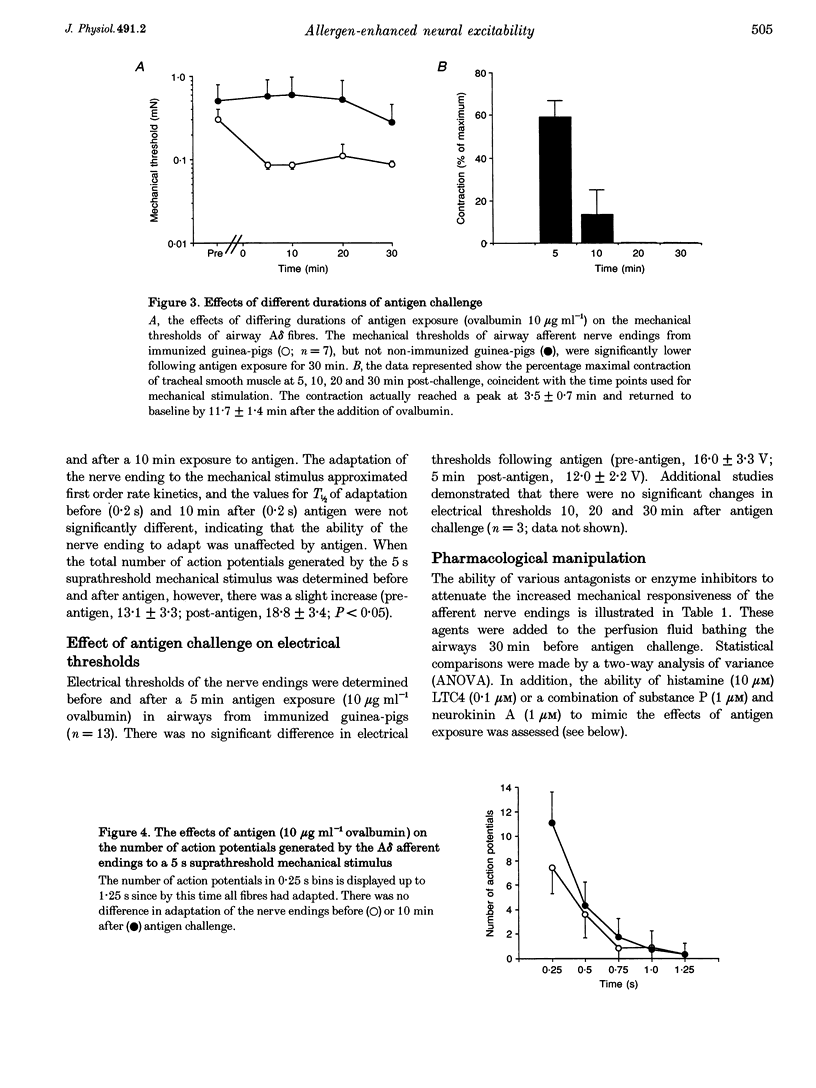
1. The trachea, larynx and main bronchi with the right vagus nerve and nodose ganglion were isolated from guinea-pigs passively immunized 24 h previously with serum containing anti-ovalbumin antibody. 2. The airways were placed in one compartment of a Perspex chamber for recording of isometric tension while the nodose ganglion and attached vagus nerve were pulled into another compartment. Action potentials arriving from single airway afferent nerve endings were monitored extracellularly using a glass microelectrode positioned near neuronal cell bodies in the ganglion. Mechanosensitivity of the nerve endings was quantified using calibrated von Frey filaments immediately before and after exposure to antigen (10 micrograms ml-1 ovalbumin). 3. Ten endings responded to the force exerted by the lowest filament (0.078 mN) and were not further investigated. In airways from thirteen immunized guinea-pigs, the mechanical sensitivity of A delta afferent fibres (conduction velocity = 4.3 +/- 0.6 m s-1) was enhanced 4.1 +/- 0.9-fold following airway exposure to antigen (P < 0.005). Mechanical sensitivities of afferent fibres (conduction velocity = 4.3 +/- 0.6 m s-1) from non-immunized control guinea-pig airways were unaffected by antigen (n = 13). 4. Antigen did not overtly cause action potential generation except in one instance when the receptive field was located over the smooth muscle. This ending also responded to methacholine suggesting that spatial changes in the receptive field, induced by muscle contraction, were responsible for the activation. 5. The mediators responsible for these effects are unknown, although histamine, prostaglandins, leukotrienes and tachykinins do not appear to be essential. The increase in mechanical responsiveness was not associated with the smooth muscle contraction since leukotriene C4, histamine and tachykinins, which all caused a similar contraction to antigen, did not affect mechanical thresholds. Moreover, the antigen-induced increases in excitability persisted beyond the duration of the smooth muscle contraction. 6. These results demonstrate that antigen-antibody-mediated inflammatory processes may enhance the excitability of vagal afferent nerve terminals projecting from the airway and thus may contribute to the pathophysiology of allergic airway diseases.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. K., 3rd, Lichtenstein L. In vitro studies of antigen-induced bronchospasm: effect of antihistamine and SRS-A antagonist on response of sensitized guinea pig and human airways to antigen. J Immunol. 1979 Feb;122(2):555–562. [PubMed] [Google Scholar]
- Baraniuk J. N., Silver P. B., Kaliner M. A., Barnes P. J. Effects of ipratropium bromide on bradykinin nasal provocation in chronic allergic rhinitis. Clin Exp Allergy. 1994 Aug;24(8):724–729. doi: 10.1111/j.1365-2222.1994.tb00982.x. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Silver P. B., Kaliner M. A., Barnes P. J. Perennial rhinitis subjects have altered vascular, glandular, and neural responses to bradykinin nasal provocation. Int Arch Allergy Immunol. 1994;103(2):202–208. doi: 10.1159/000236628. [DOI] [PubMed] [Google Scholar]
- Bergren D. R., Sampson S. R. Characterization of intrapulmonary, rapidly adapting receptors of guinea pigs. Respir Physiol. 1982 Jan;47(1):83–95. doi: 10.1016/0034-5687(82)90094-9. [DOI] [PubMed] [Google Scholar]
- Bradley G. W., Scheurmier N. The transduction properties of tracheal stretch receptors in vitro. Respir Physiol. 1977 Dec;31(3):365–375. doi: 10.1016/0034-5687(77)90079-2. [DOI] [PubMed] [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994 Jan;74(1):95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Hong K. A., Langford L. A., Schaible H. G., Schmidt R. F. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Res. 1983 Aug 1;272(1):185–188. doi: 10.1016/0006-8993(83)90379-7. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Austen K. F. Pulmonary response to antigen infusion in the sensitized guinea pig: modification by atropine. J Appl Physiol. 1975 Dec;39(6):916–919. doi: 10.1152/jappl.1975.39.6.916. [DOI] [PubMed] [Google Scholar]
- Ellis J. L., Undem B. J. Antigen-induced enhancement of noncholinergic contractile responses to vagus nerve and electrical field stimulation in guinea pig isolated trachea. J Pharmacol Exp Ther. 1992 Aug;262(2):646–653. [PubMed] [Google Scholar]
- Ellis J. L., Undem B. J. Role of peptidoleukotrienes in capsaicin-sensitive sensory fibre-mediated responses in guinea-pig airways. J Physiol. 1991 May;436:469–484. doi: 10.1113/jphysiol.1991.sp018561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. J., Barnes P. J., Urban L., Dray A. An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J Physiol. 1993 Sep;469:21–35. doi: 10.1113/jphysiol.1993.sp019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold W. M., Kessler G. F., Yu D. Y. Role of vagus nerves in experimental asthma in allergic dogs. J Appl Physiol. 1972 Dec;33(6):719–725. doi: 10.1152/jappl.1972.33.6.719. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990 Jun;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski W., Widdicombe J. G. The role of the vagus nerves in the respiratory and circulatory reactions to anaphylaxis in rabbits. J Physiol. 1969 Apr;201(2):293–304. doi: 10.1113/jphysiol.1969.sp008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W., Fischer A., Kurkowski R., Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992 Aug;49(3):715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Morton R. F. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respir Physiol. 1993 Jul;93(1):83–96. doi: 10.1016/0034-5687(93)90070-q. [DOI] [PubMed] [Google Scholar]
- Longley J., Duffy T. P., Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995 Apr;32(4):545–564. doi: 10.1016/0190-9622(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Mei N. Disposition anatomique et propriétés électrophysiologiques des neurones sensitifs vagaux chez le chat. Exp Brain Res. 1970;11(5):465–479. [PubMed] [Google Scholar]
- Mills J. E., Sellick H., Widdicombe J. G. Activity of lung irritant receptors in pulmonary microembolism, anaphylaxis and drug-induced bronchoconstrictions. J Physiol. 1969 Aug;203(2):337–357. doi: 10.1113/jphysiol.1969.sp008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. C., Undem B. J., Weinreich D. Influence of antigen on membrane properties of guinea pig bronchial ganglion neurons. J Appl Physiol (1985) 1991 Sep;71(3):970–976. doi: 10.1152/jappl.1991.71.3.970. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Bevan S., Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull. 1991 Jul;47(3):534–548. doi: 10.1093/oxfordjournals.bmb.a072491. [DOI] [PubMed] [Google Scholar]
- Riccio M. M., Reynolds C. J., Hay D. W., Proud D. Effects of intranasal administration of endothelin-1 to allergic and nonallergic individuals. Am J Respir Crit Care Med. 1995 Dec;152(6 Pt 1):1757–1764. doi: 10.1164/ajrccm.152.6.8520734. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B. Mast cells: function and contents. Curr Opin Immunol. 1994 Feb;6(1):91–97. doi: 10.1016/0952-7915(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Sellick H., Widdicombe J. G. Stimulation of lung irritant receptors by cigarette smoke, carbon dust, and histamine aerosol. J Appl Physiol. 1971 Jul;31(1):15–19. doi: 10.1152/jappl.1971.31.1.15. [DOI] [PubMed] [Google Scholar]
- Undem B. J., Buckner C. K., Harley P., Graziano F. M. Smooth muscle contraction and release of histamine and slow-reacting substance of anaphylaxis in pulmonary tissues isolated from guinea pigs passively sensitized with IgG1 or IgE antibodies. Am Rev Respir Dis. 1985 Feb;131(2):260–266. doi: 10.1164/arrd.1985.131.2.260. [DOI] [PubMed] [Google Scholar]
- Undem B. J., Hubbard W., Weinreich D. Immunologically induced neuromodulation of guinea pig nodose ganglion neurons. J Auton Nerv Syst. 1993 Jul;44(1):35–44. doi: 10.1016/0165-1838(93)90376-6. [DOI] [PubMed] [Google Scholar]
- Vidruk E. H., Hahn H. L., Nadel J. A., Sampson S. R. Mechanisms by which histamine stimulates rapidly adapting receptors in dog lungs. J Appl Physiol Respir Environ Exerc Physiol. 1977 Sep;43(3):397–402. doi: 10.1152/jappl.1977.43.3.397. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. I. Mast cells and airway inflammation in asthma. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 2):S39–S41. doi: 10.1164/ajrccm/150.5_Pt_2.S39. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Wonderlin W. F. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol. 1987 Dec;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]


