Abstract
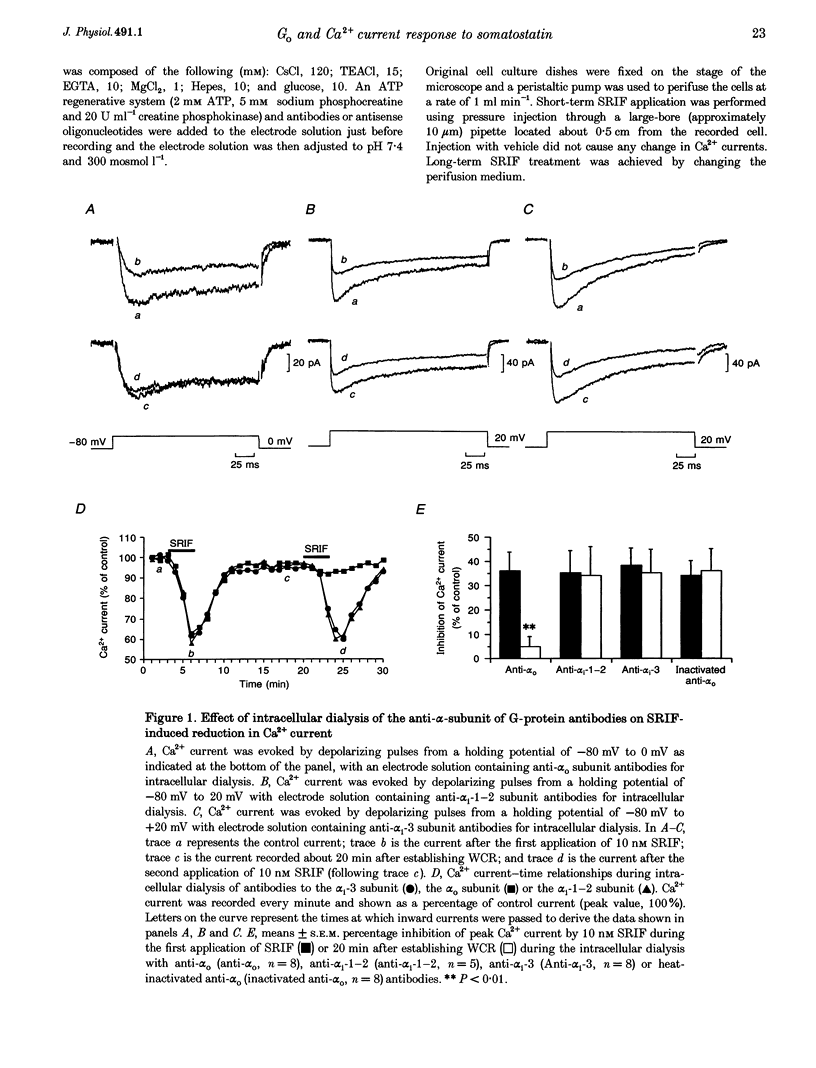
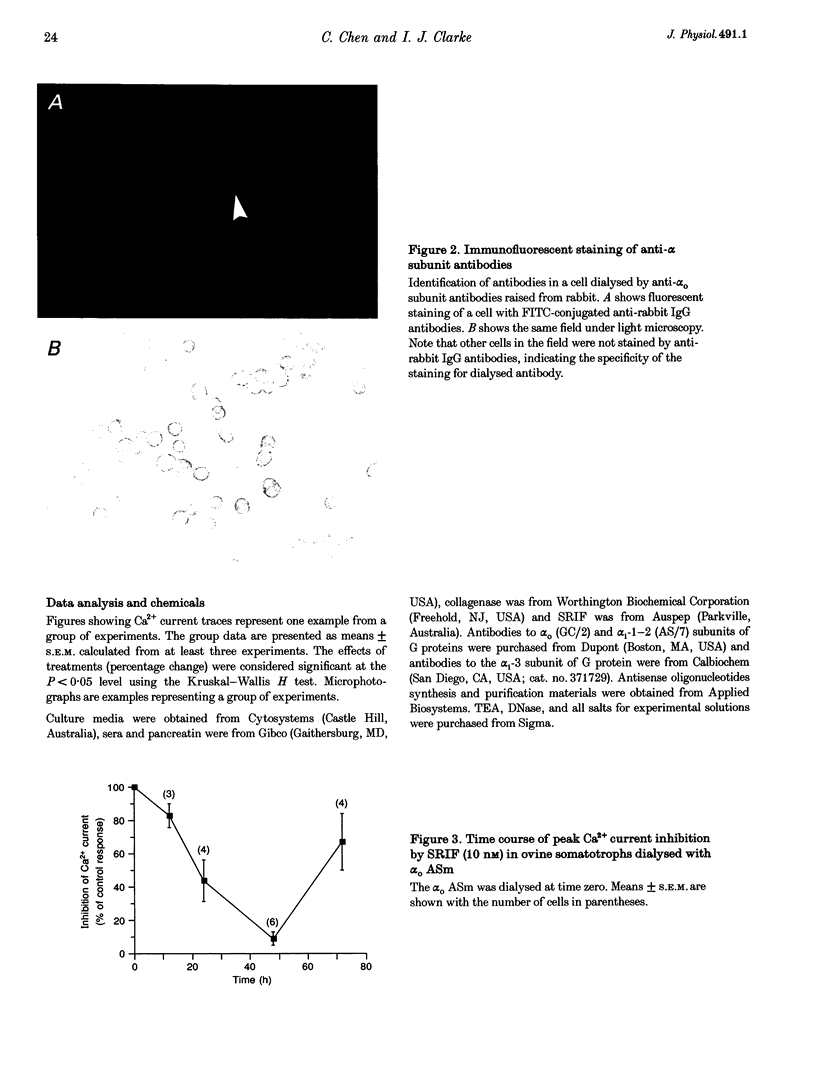
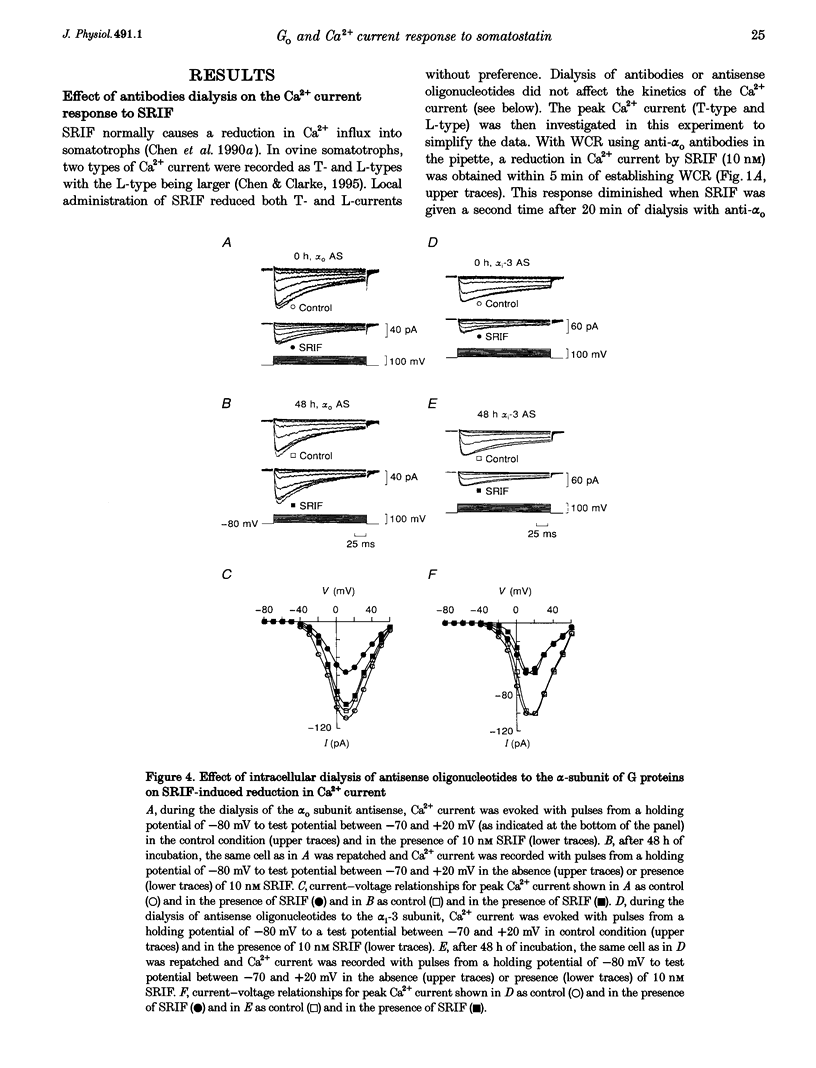
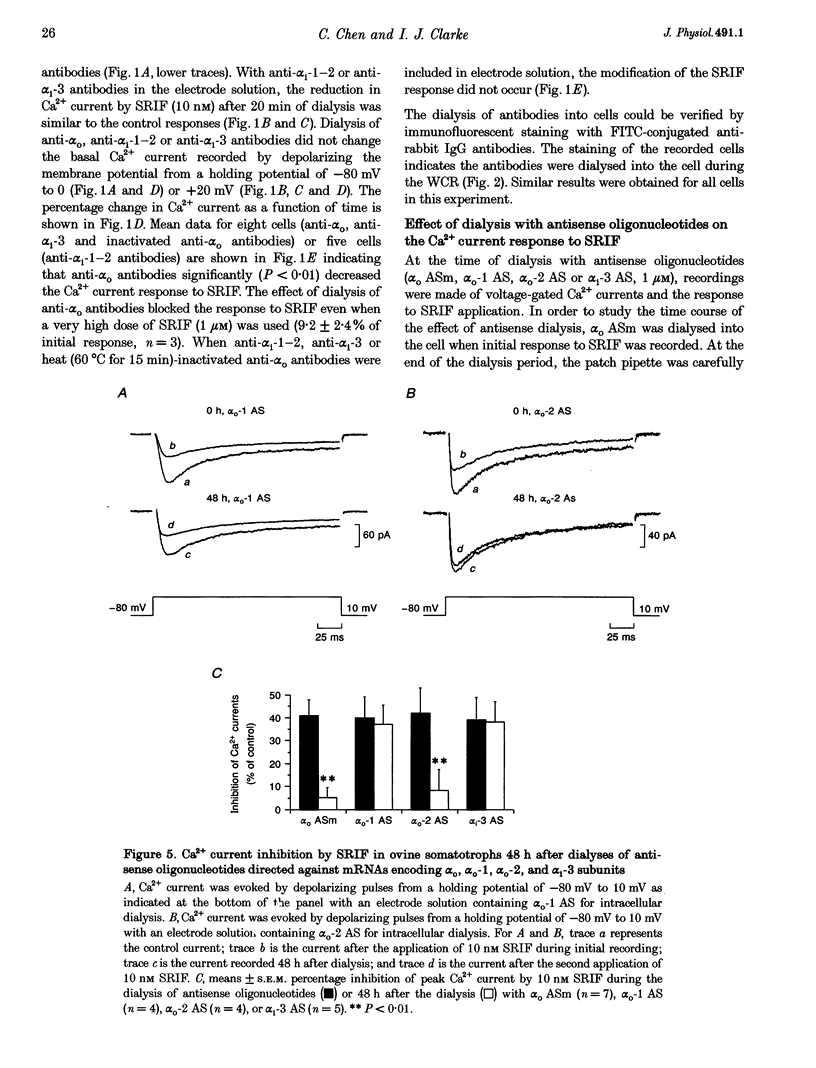
1. Somatotroph-enriched cells (up to 85%) were obtained from ovine pituitary glands by means of collagenase dissociation and Percoll-gradient centrifugation. Further identification was based on the reduction in Ca2+ currents by 10 nM somatostatin (SRIF). 2. The whole-cell configuration of the patch-clamp technique was employed to study the membrane Ca2+ currents with K+ ions replaced by Cs+ and the addition of K+ and Na+ channel blockers in bath and pipette solutions. 3. A significant reduction in Ca2+ currents was obtained in response to local application of SRIF (10 nM) but vehicle application had no effect. 4. Intracellular dialysis of antibodies to alpha(o), alpha(i)-1-2, or alpha(i)-3 subunits of G proteins into the cells via patch-clamp pipettes was confirmed by immunofluorescent staining of the antibodies. Antibody dialysis did not modify resting voltage-gated Ca2+ currents across the cell membrane. 5. Dialysis of anti-alpha(o) antibodies significantly attenuated the reduction in Ca2+ currents that was obtained upon application of 10 or 100 nM SRIF. Dialysis of neither anti-alpha(i)-1-2 nor anti-alpha(i)-3 antibodies diminished the effect of SRIF on Ca2+ currents. 6. Intracellular dialysis of antisense oligonucleotides directed against the alpha(o) subunit mRNA (alpha(o) ASm, for alpha(o) common) or against the alpha(i)-3 subunit mRNA (alpha(i)-3 AS) blocked expression of alpha(o) or alpha(i)-3 subunits in the cells, respectively, as assessed by fluorescent staining with anti-alpha(o) or anti-alpha(i)-3 antibodies 48 h after dialysis. 7. Dialysis of alpha(o) ASm, but not alpha(i)-3 AS, significantly diminished the inhibitory effect of SRIF on Ca2+ currents. This effect of alpha(o) ASm dialysis occurred within 12 h after dialysis and reached a maximum at 48 h; partial recovery was seen at 72 h. 8. Antisense oligonucleotides specific for alpha(o)-1 (alpha(o)-1 AS) or alpha(o)-2 (alpha(o)-2 AS) were dialysed into somatotrophs and only alpha(o)-2 AS significantly attenuated the inhibition of Ca2+ currents by SRIF. 9. We conclude that the G(o)-2 protein mediates the effect of SRIF on Ca2+ currents in ovine somatotrophs in primary culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baertschi A. J., Audigier Y., Lledo P. M., Israel J. M., Bockaert J., Vincent J. D. Dialysis of lactotropes with antisense oligonucleotides assigns guanine nucleotide binding protein subtypes to their channel effectors. Mol Endocrinol. 1992 Dec;6(12):2257–2265. doi: 10.1210/mend.6.12.1337149. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Reisine T. Molecular biology of somatostatin receptors. Trends Neurosci. 1993 Jan;16(1):34–38. doi: 10.1016/0166-2236(93)90050-v. [DOI] [PubMed] [Google Scholar]
- Chen C., Clarke I. J. Modulation of Ca2+ influx in the ovine somatotroph by growth hormone-releasing factor. Am J Physiol. 1995 Feb;268(2 Pt 1):E204–E212. doi: 10.1152/ajpendo.1995.268.2.E204. [DOI] [PubMed] [Google Scholar]
- Chen C., Heyward P., Zhang J., Wu D., Clarke I. J. Voltage-dependent potassium currents in ovine somatotrophs and their function in growth hormone secretion. Neuroendocrinology. 1994 Jan;59(1):1–9. doi: 10.1159/000126631. [DOI] [PubMed] [Google Scholar]
- Chen C., Israel J. M., Vincent J. D. Electrophysiological responses to somatostatin of rat hypophysial cells in somatotroph-enriched primary cultures. J Physiol. 1989 Jan;408:493–510. doi: 10.1113/jphysiol.1989.sp017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang J., Vincent J. D., Israel J. M. Two types of voltage-dependent calcium current in rat somatotrophs are reduced by somatostatin. J Physiol. 1990 Jun;425:29–42. doi: 10.1113/jphysiol.1990.sp018090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum J., Enjalbert A., Krantic S., Musset F., Bertrand P., Rasolonjanahary R., Shu C., Kordon C. Somatostatin receptors on pituitary somatotrophs, thyrotrophs, and lactotrophs: pharmacological evidence for loose coupling to adenylate cyclase. Endocrinology. 1987 Dec;121(6):2177–2185. doi: 10.1210/endo-121-6-2177. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Somatostatin cyclic octapeptide analogs which preferentially bind to SOMa receptors block a calcium current in rat superior cervical ganglion neurons. Neurosci Lett. 1989 Jan 30;96(3):283–288. doi: 10.1016/0304-3940(89)90392-3. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991 Sep 5;353(6339):43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Meriney S. D., Gray D. B., Pilar G. R. Somatostatin-induced inhibition of neuronal Ca2+ current modulated by cGMP-dependent protein kinase. Nature. 1994 May 26;369(6478):336–339. doi: 10.1038/369336a0. [DOI] [PubMed] [Google Scholar]
- Nussinovitch I. Somatostatin inhibits two types of voltage-activated calcium currents in rat growth-hormone secreting cells. Brain Res. 1989 Dec 11;504(1):136–138. doi: 10.1016/0006-8993(89)91610-7. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Shen K. Z., North R. A., Tatsumi H. Inhibition of calcium currents by noradrenaline, somatostatin and opioids in guinea-pig submucosal neurones. J Physiol. 1990 Dec;431:585–608. doi: 10.1113/jphysiol.1990.sp018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Rens-Domiano S., Breder C. D., Law S. F., Saper C. B., Reisine T., Bell G. I. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992 Oct 5;267(28):20422–20428. [PubMed] [Google Scholar]
- Yatani A., Codina J., Sekura R. D., Birnbaumer L., Brown A. M. Reconstitution of somatostatin and muscarinic receptor mediated stimulation of K+ channels by isolated GK protein in clonal rat anterior pituitary cell membranes. Mol Endocrinol. 1987 Apr;1(4):283–289. doi: 10.1210/mend-1-4-283. [DOI] [PubMed] [Google Scholar]




