Abstract
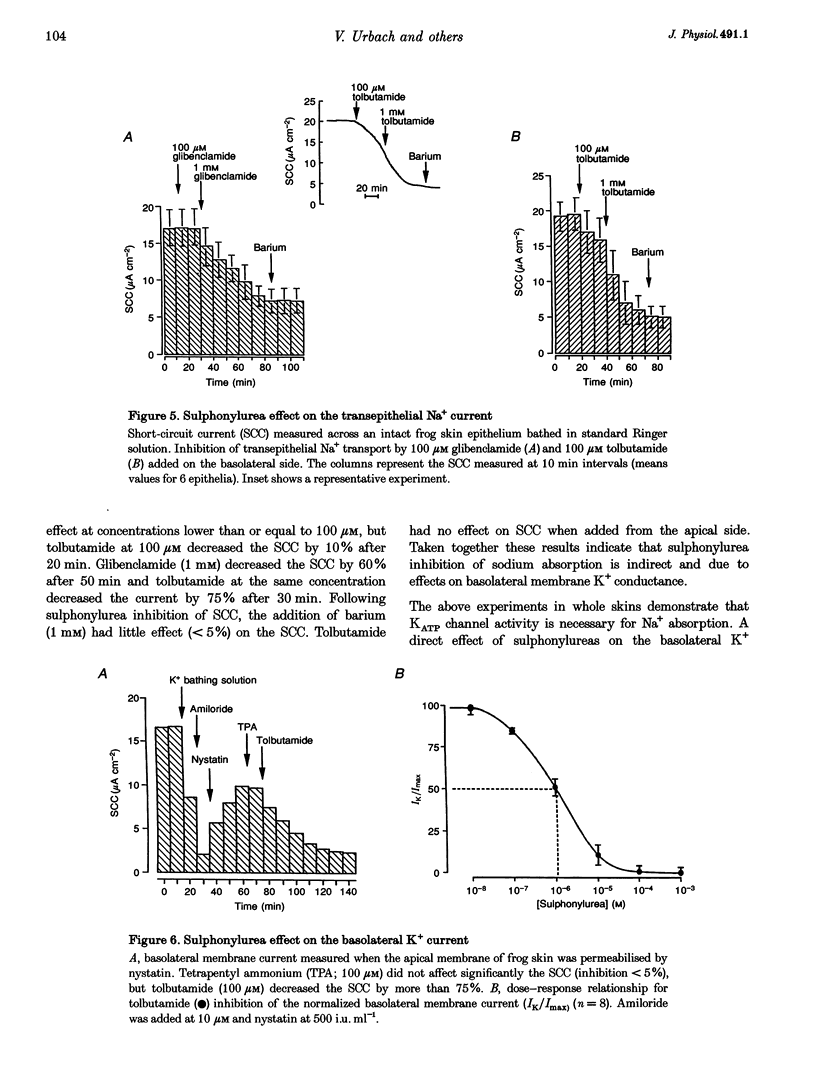
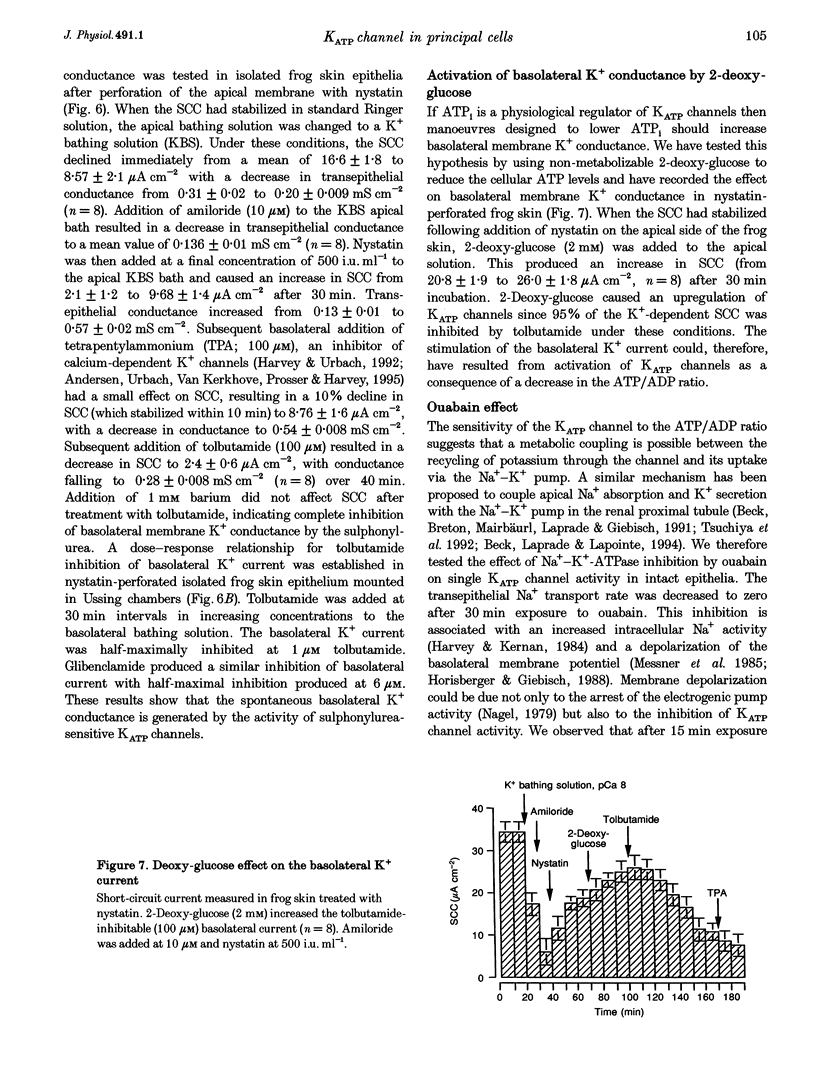
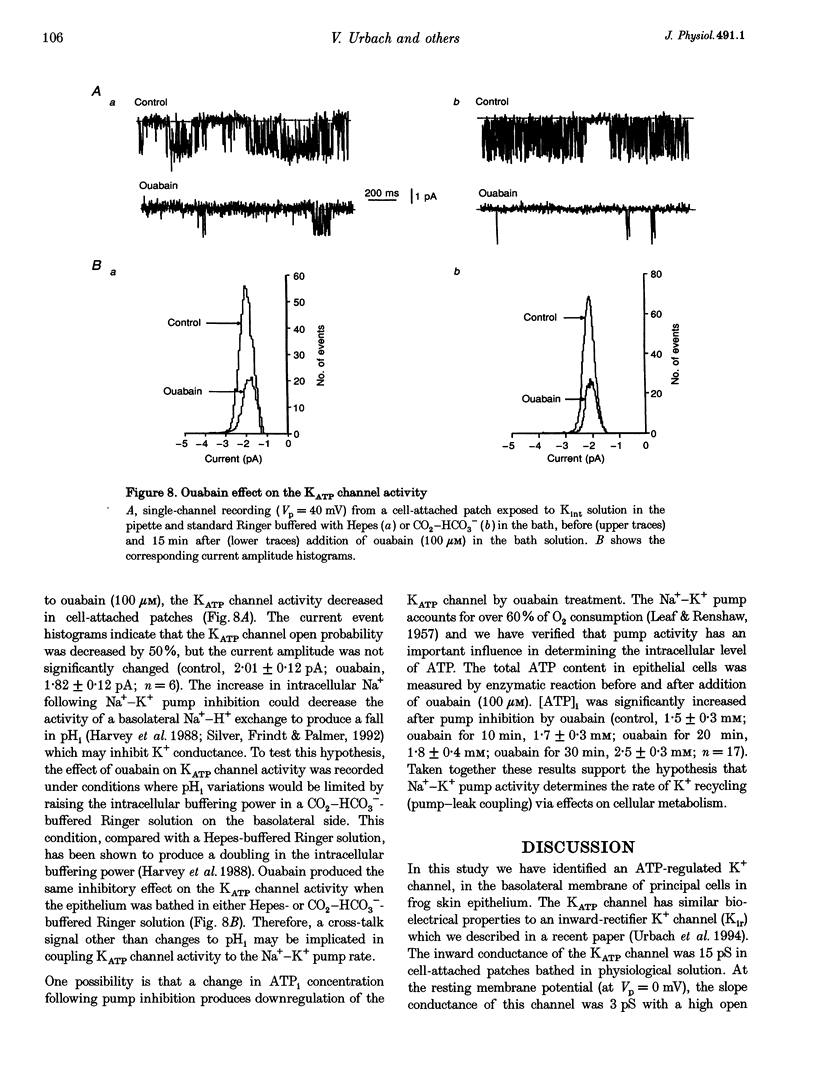
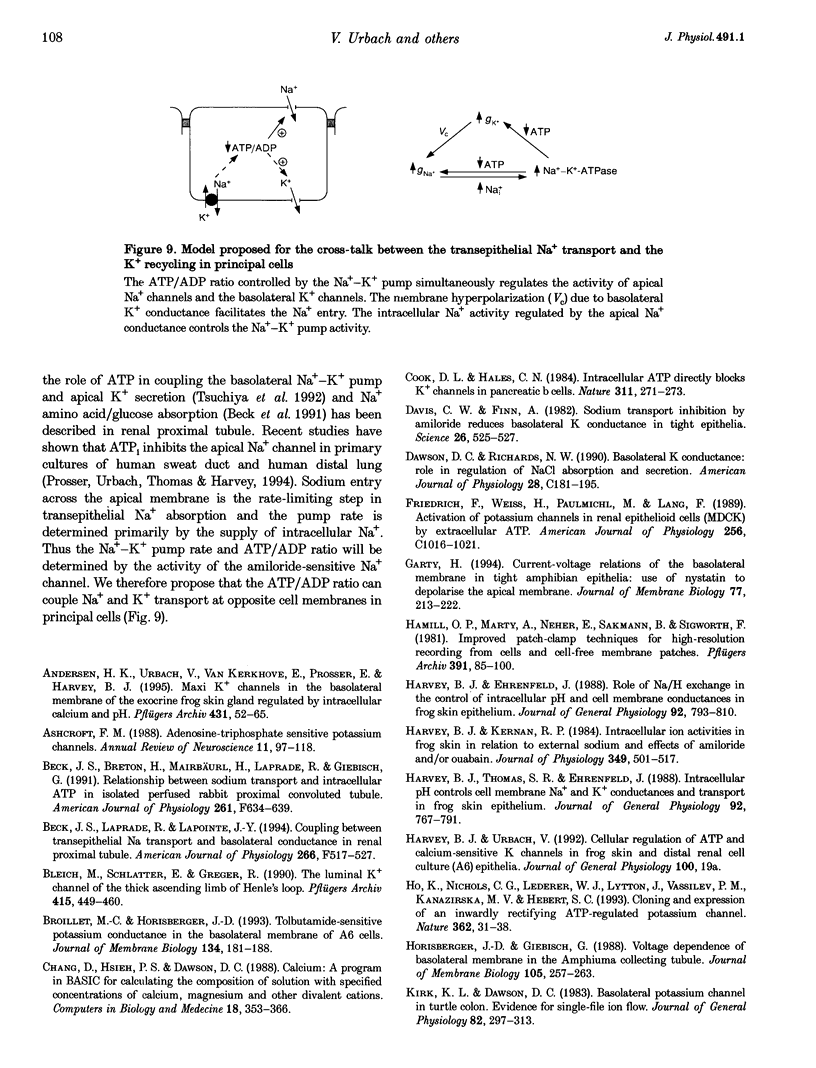
Isolated frog skin epithelium, mounted in an Ussing chamber and bathed in standard NaCl Ringer solution, recycles K+ across the basolateral membrane of principal cells through an inward-rectifier K+ channel (Kir) operating in parallel with a Na+-K+-ATPase pump. Here we report on the metabolic control of the Kir channel using patch clamping, short-circuit current measurement and enzymatic determination of cellular (ATP (ATPi). 2. The constitutively active Kir channel in the basolateral membrane has the characteristics of an ATP-regulated K+ channel and is now classed as a KATP channel. In excised inside-out patches the open probability (Po) of KATP channels was reduced by ATPi with half-maximum inhibition at an ATPi concentration of 50 microM. 3. ATPi measured (under normal Na+ transport conditions) with luciferin-luciferase was 1.50 +/- 0.23 mM (mean +/- S.E.M.; range, 0.4-3.3 mM n = 11). Thus the KATP channel would be expected to be inactive in intact cells if ATPi was the sole regulator of channel activity. KATP channels which were inactivated by 1 mM ATPi in excised patches could be reactivated by addition of 100 microM ADP on the cytosolic side. When added alone, ADP blocks this channel with half-maximal inhibition at [ADPi] > 5 mM. 4. Sulphonylureas inhibit single KATP channels in cell-attached patches as well as the total basolateral K+ current measured in frog skin epithelia perforated with nystatin on the apical side. 5. Na+-K+-ATPase activity is a major determinant of cytosolic ATP. Blocking the pump activity with ouabain produced a time-dependent increase in ATPi and reduced the open probability of KATP channels in cell-attached membranes. 6. We conclude that the ratio of ATP/ADP is an important metabolic coupling factor between the rate of Na+-K+ pumping and K+ recycling.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K., Urbach V., Van Kerkhove E., Prosser E., Harvey B. J. Maxi K+ channels in the basolateral membrane of the exocrine frog skin gland regulated by intracellular calcium and pH. Pflugers Arch. 1995 Nov;431(1):52–65. doi: 10.1007/BF00374377. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Beck J. S., Breton S., Mairbäurl H., Laprade R., Giebisch G. Relationship between sodium transport and intracellular ATP in isolated perfused rabbit proximal convoluted tubule. Am J Physiol. 1991 Oct;261(4 Pt 2):F634–F639. doi: 10.1152/ajprenal.1991.261.4.F634. [DOI] [PubMed] [Google Scholar]
- Beck J. S., Laprade R., Lapointe J. Y. Coupling between transepithelial Na transport and basolateral K conductance in renal proximal tubule. Am J Physiol. 1994 Apr;266(4 Pt 2):F517–F527. doi: 10.1152/ajprenal.1994.266.4.F517. [DOI] [PubMed] [Google Scholar]
- Bleich M., Schlatter E., Greger R. The luminal K+ channel of the thick ascending limb of Henle's loop. Pflugers Arch. 1990 Jan;415(4):449–460. doi: 10.1007/BF00373623. [DOI] [PubMed] [Google Scholar]
- Broillet M. C., Horisberger J. D. Tolbutamide-sensitive potassium conductance in the basolateral membrane of A6 cells. J Membr Biol. 1993 Jun;134(3):181–188. doi: 10.1007/BF00234499. [DOI] [PubMed] [Google Scholar]
- Chang D., Hsieh P. S., Dawson D. C. Calcium: a program in BASIC for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Comput Biol Med. 1988;18(5):351–366. doi: 10.1016/0010-4825(88)90022-4. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Sodium transport inhibition by amiloride reduces basolateral membrane potassium conductance in tight epithelia. Science. 1982 Apr 30;216(4545):525–527. doi: 10.1126/science.7071599. [DOI] [PubMed] [Google Scholar]
- Friedrich F., Weiss H., Paulmichl M., Lang F. Activation of potassium channels in renal epithelioid cells (MDCK) by extracellular ATP. Am J Physiol. 1989 May;256(5 Pt 1):C1016–C1021. doi: 10.1152/ajpcell.1989.256.5.C1016. [DOI] [PubMed] [Google Scholar]
- Garty H. Current-voltage relations of the basolateral membrane in tight amphibian epithelia: use of nystatin to depolarize the apical membrane. J Membr Biol. 1984;77(3):213–222. doi: 10.1007/BF01870570. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey B. J., Ehrenfeld J. Role of Na+/H+ exchange in the control of intracellular pH and cell membrane conductances in frog skin epithelium. J Gen Physiol. 1988 Dec;92(6):793–810. doi: 10.1085/jgp.92.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. J., Kernan R. P. Intracellular ion activities in frog skin in relation to external sodium and effects of amiloride and/or ouabain. J Physiol. 1984 Apr;349:501–517. doi: 10.1113/jphysiol.1984.sp015170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. J., Thomas S. R., Ehrenfeld J. Intracellular pH controls cell membrane Na+ and K+ conductances and transport in frog skin epithelium. J Gen Physiol. 1988 Dec;92(6):767–791. doi: 10.1085/jgp.92.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Horisberger J. D., Giebisch G. Voltage dependence of the basolateral membrane conductance in the Amphiuma collecting tubule. J Membr Biol. 1988 Nov;105(3):257–263. doi: 10.1007/BF01871002. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Dawson D. C. Basolateral potassium channel in turtle colon. Evidence for single-file ion flow. J Gen Physiol. 1983 Sep;82(3):297–313. doi: 10.1085/jgp.82.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAF A., RENSHAW A. Ion transport and respiration of isolated frog skin. Biochem J. 1957 Jan;65(1):82–90. doi: 10.1042/bj0650082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner G., Wang W., Paulmichl M., Oberleithner H., Lang F. Ouabain decreases apparent potassium-conductance in proximal tubules of the amphibian kidney. Pflugers Arch. 1985 May;404(2):131–137. doi: 10.1007/BF00585408. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Kubota T., Yamaguchi J., Fujimoto M. Regulation of inwardly rectifying K+ channels by intracellular pH in opossum kidney cells. Pflugers Arch. 1990 Apr;416(1-2):138–143. doi: 10.1007/BF00370235. [DOI] [PubMed] [Google Scholar]
- Rick R. pHi determines rate of sodium transport in frog skin: results of a new method to determine pHi. Am J Physiol. 1994 Mar;266(3 Pt 2):F367–F374. doi: 10.1152/ajprenal.1994.266.3.F367. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. B., Frindt G., Palmer L. G. Regulation of principal cell pH by Na/H exchange in rabbit cortical collecting tubule. J Membr Biol. 1992 Jan;125(1):13–24. doi: 10.1007/BF00235794. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Frindt G., Windhager E. E., Palmer L. G. Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. Am J Physiol. 1993 Mar;264(3 Pt 2):F557–F564. doi: 10.1152/ajprenal.1993.264.3.F557. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Wang W., Giebisch G., Welling P. A. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach V., van Kerkhove E., Harvey B. J. Inward-rectifier potassium channels in basolateral membranes of frog skin epithelium. J Gen Physiol. 1994 Apr;103(4):583–604. doi: 10.1085/jgp.103.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Giebisch G. Dual effect of adenosine triphosphate on the apical small conductance K+ channel of the rat cortical collecting duct. J Gen Physiol. 1991 Jul;98(1):35–61. doi: 10.1085/jgp.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J Physiol. 1992 Sep;455:247–269. doi: 10.1113/jphysiol.1992.sp019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zünkler B. J., Lenzen S., Männer K., Panten U., Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]