Abstract
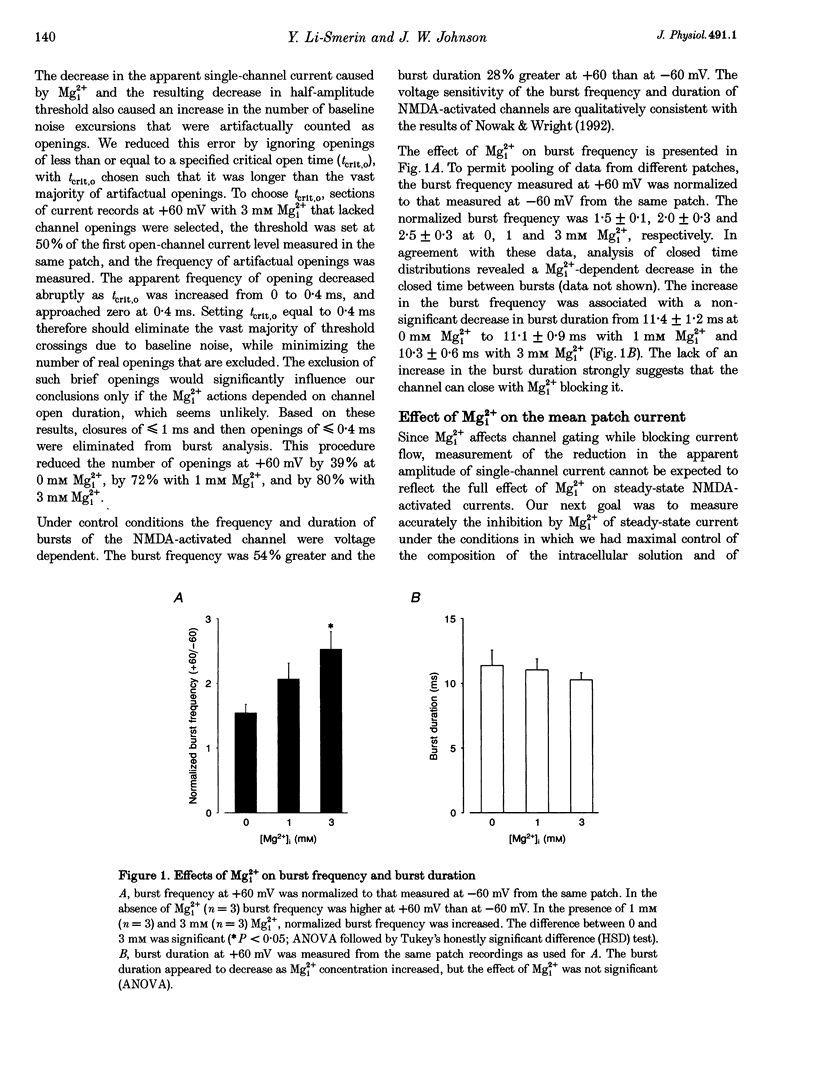
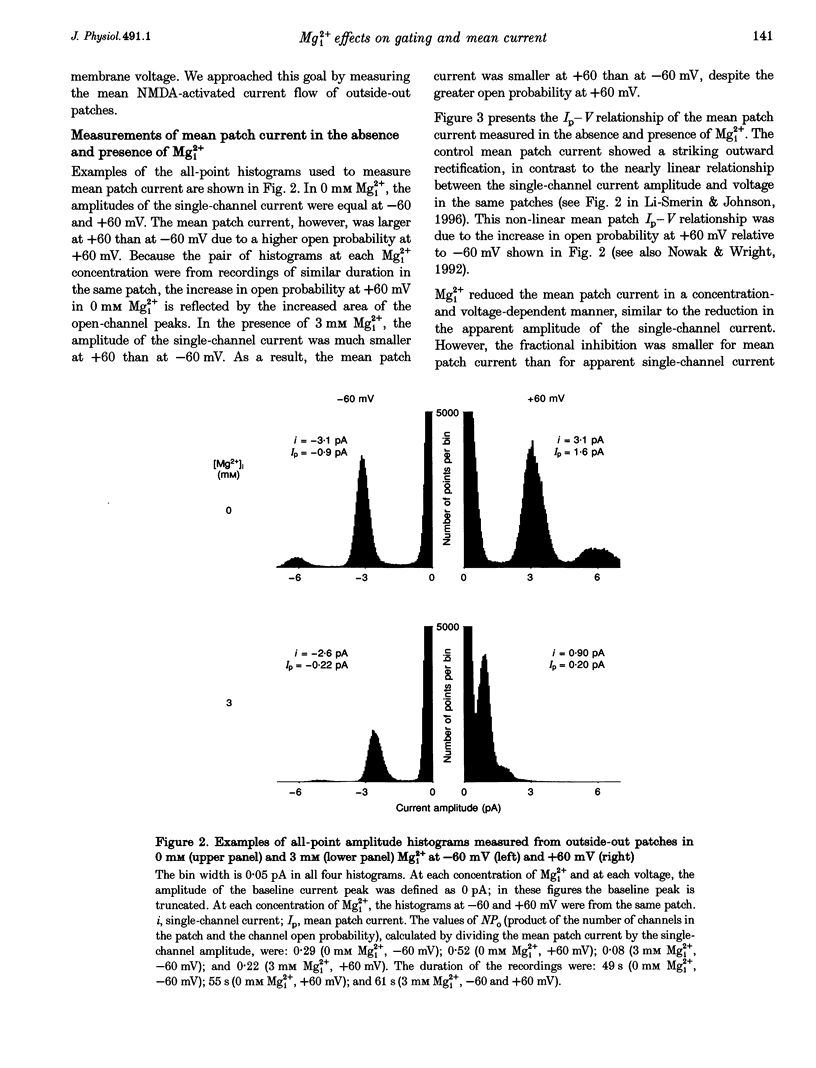
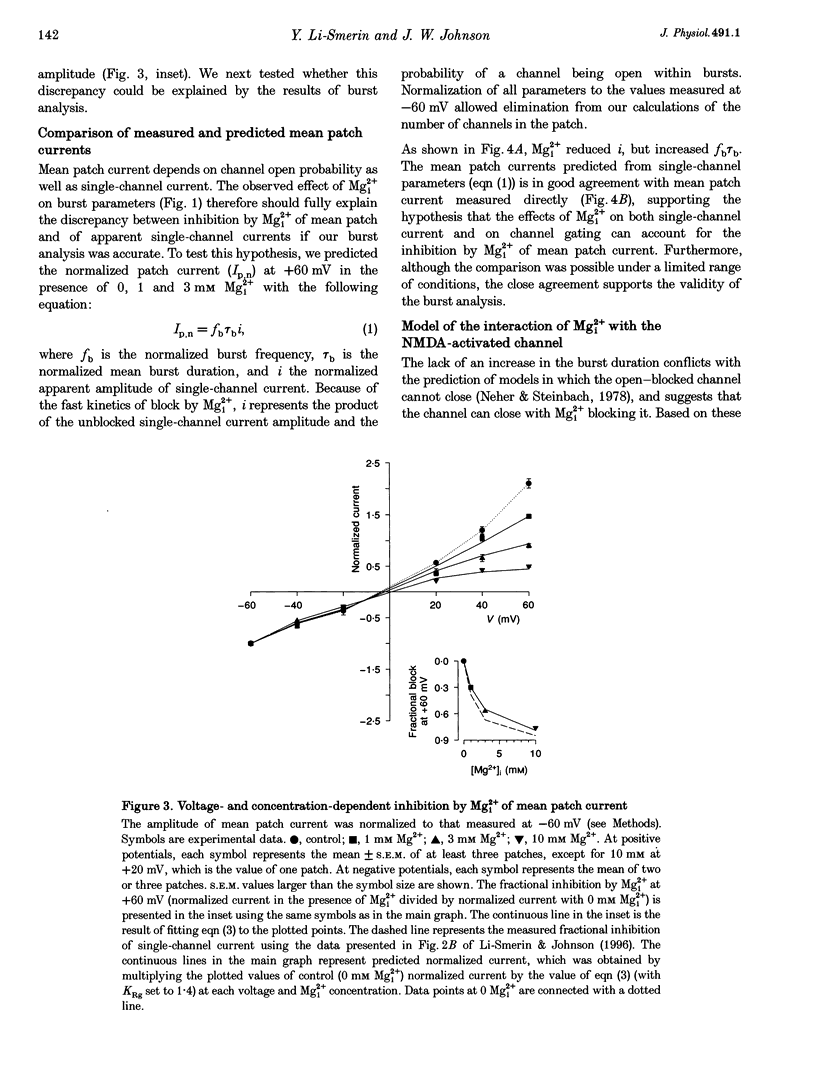
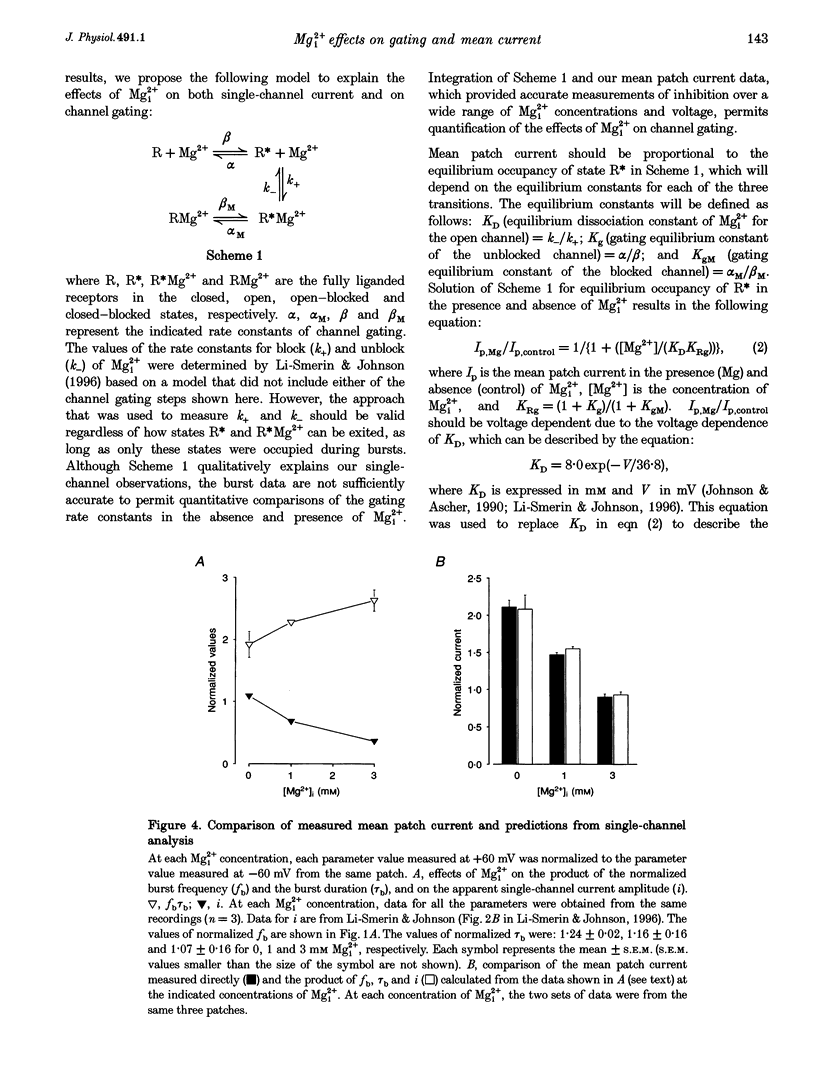
1. The effects of intracellular Mg2+ (Mgi2+) on the single N-methyl-D-aspartate (NMDA)-activated channel burst duration and frequency and on the mean NMDA-activated patch current were studied in outside-out patches from cultured rat cortical neurons. The inhibition by Mgi2+ of mean patch and whole-cell currents were compared, and some possible explanations for the observed differences were investigated. 2. The burst duration at +60 mV did not depend on Mgi2+ concentration, suggesting that the channel can close when blocked by Mgi2+. The number of bursts per second increased significantly in the presence of Mgi2+, suggesting that the rate of channel opening is higher when Mg2+ from the intracellular solution occupies its binding site. 3. Mgi2+ caused a voltage- and concentration-dependent inhibition of mean patch current. The inhibition is in quantitative agreement with the effects of Mgi2+ on the single-channel current and on burst parameters. 4. Based on the effects of Mgi2+ on burst parameters and on single-channel current, a four-state model in which the NMDA-activated channel can close while blocked by Mgi2+ is proposed. By fitting the model to the mean patch current data, we estimate that the rate of channel opening is increased by a factor of 1.4 when Mgi2+ occupies the channel. This estimation provides evidence that occupancy of the NMDA-activated channel by Mgi2+ destabilizes the closed state. 5. Mgi2+ reduced NMDA-activated whole-cell currents in a voltage- and concentration-dependent manner. However, normalized whole-cell and mean patch currents at positive voltages differed in two significant respects. First, when currents were recorded in a 0 Mg2+ pipette solution, whole-cell currents at positive voltages were smaller. Second, Mgi2+ appeared to inhibit whole-cell current less effectively than it inhibited mean patch current. 6. Inclusion of the Mg2+ chelators EDTA and ATP in 0 Mg2+ pipette solutions did not increase the whole-cell current measured at +60 mV. This observation suggests that the difference between normalized whole-cell and mean patch currents with 0 Mg2+ pipette solution was not due to block of whole-cell currents by residual Mgi2+. 7. When a pipette solution containing EGTA and Mg2+ was used to buffer Mgi2+, inhibition by Mgi2+ of the whole-cell current was enhanced, suggesting that the free Mg2+ concentration inside a neuron can remain below the pipette Mg2+ concentration. However, we cannot exclude other explanations for the differences between the inhibition by Mg2+ of mean patch and whole-cell currents.
Full text
PDF






Selected References
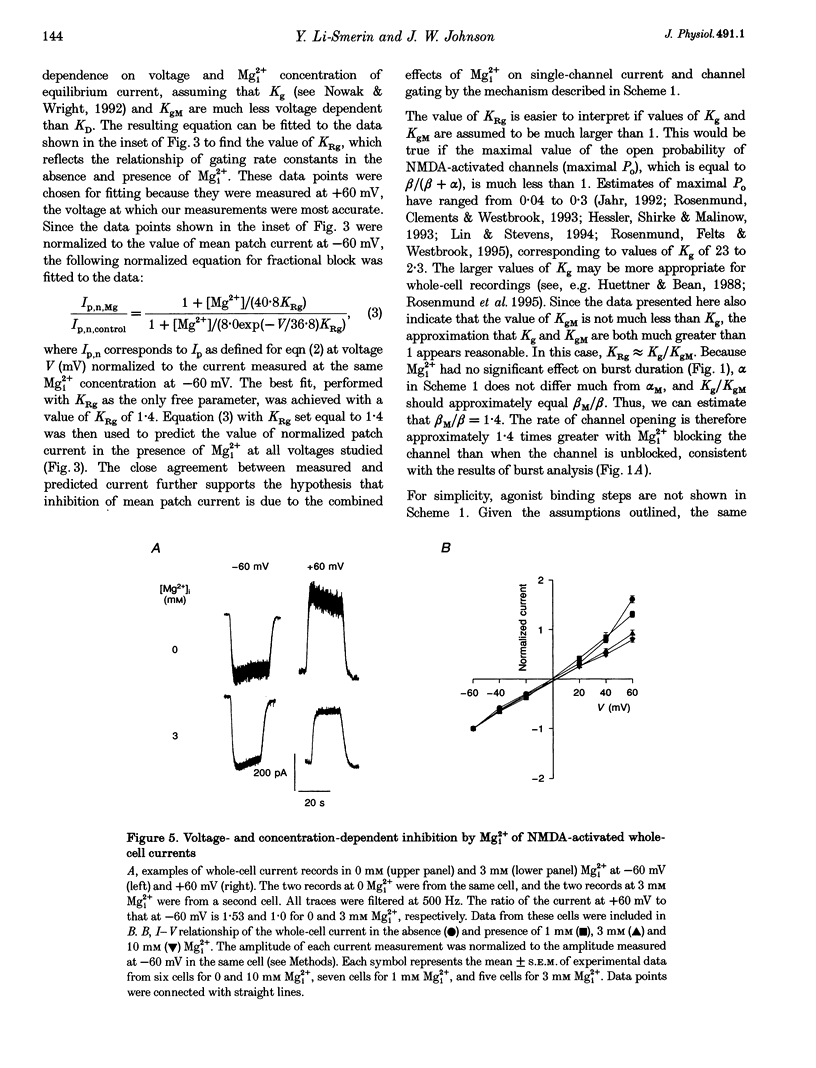
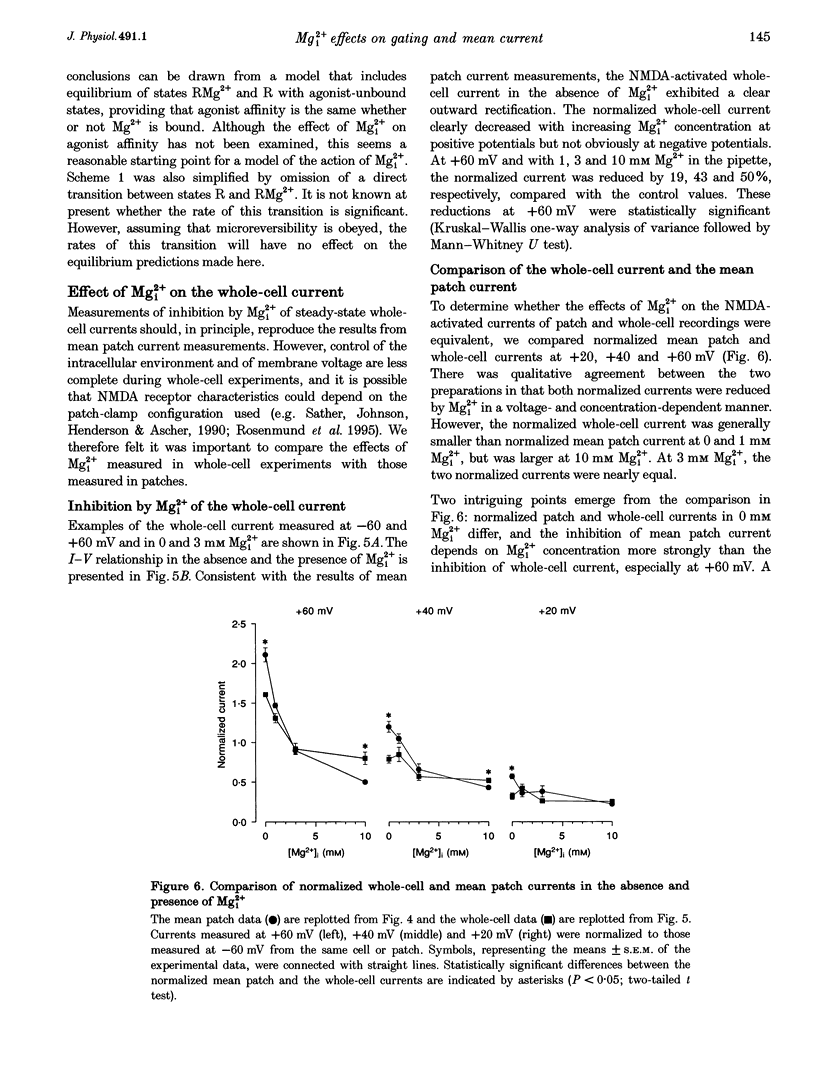
These references are in PubMed. This may not be the complete list of references from this article.
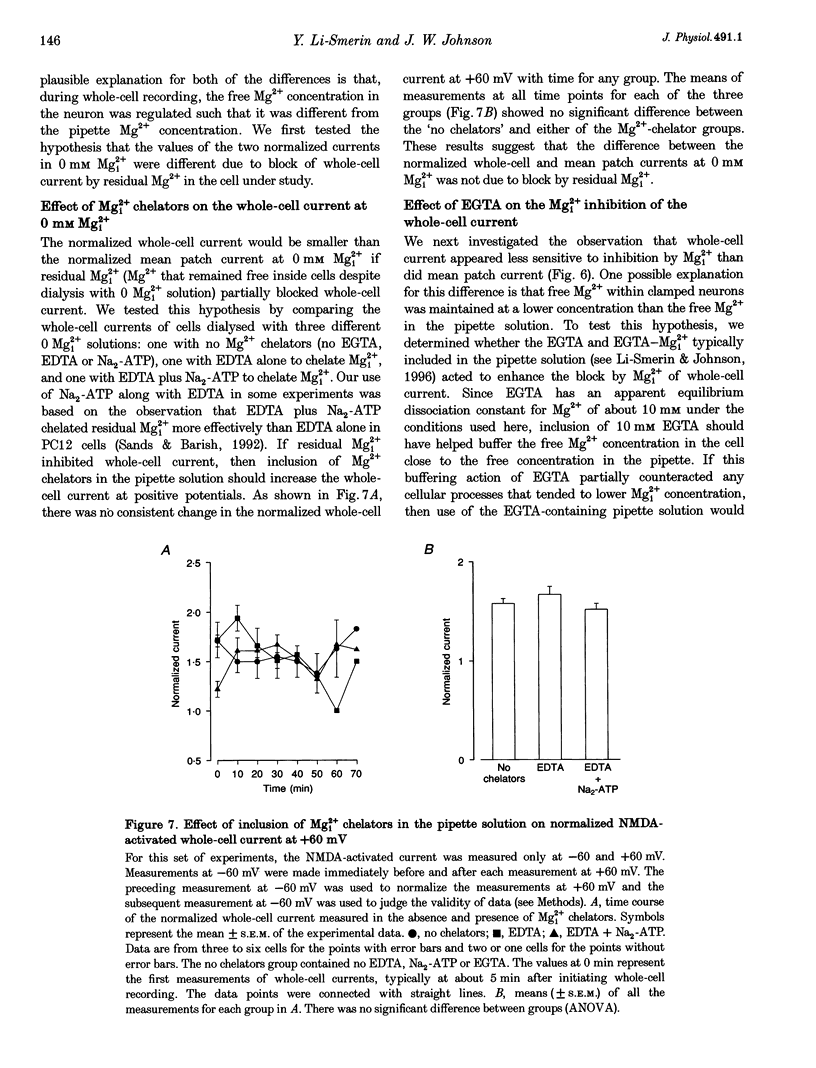
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L. Mushroom bodies and Drosophila learning. Neuron. 1993 Jul;11(1):1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. Ion effects on gating of the Ca(2+)-activated K+ channel correlate with occupancy of the pore. Biophys J. 1992 Mar;61(3):639–648. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Mechanisms of magnesium transport. Annu Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hessler N. A., Shirke A. M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993 Dec 9;366(6455):569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992 Jan 24;255(5043):470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophys J. 1990 May;57(5):1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Smerin Y., Johnson J. W. Kinetics of the block by intracellular Mg2+ of the NMDA-activated channel in cultured rat neurons. J Physiol. 1996 Feb 15;491(Pt 1):121–135. doi: 10.1113/jphysiol.1996.sp021201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Stevens C. F. Both open and closed NMDA receptor channels desensitize. J Neurosci. 1994 Apr;14(4):2153–2160. doi: 10.1523/JNEUROSCI.14-04-02153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Latorre R., Reisin I. Coupling of voltage-dependent gating and Ba++ block in the high-conductance, Ca++-activated K+ channel. J Gen Physiol. 1987 Sep;90(3):427–449. doi: 10.1085/jgp.90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Freudenrich C. C., Lieberman M. Cellular magnesium and Na/Mg exchange in heart cells. Annu Rev Physiol. 1991;53:273–287. doi: 10.1146/annurev.ph.53.030191.001421. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Pelleschi M. Multi-ion occupancy alters gating in high-conductance, Ca(2+)-activated K+ channels. J Gen Physiol. 1991 Apr;97(4):641–665. doi: 10.1085/jgp.97.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L. M., Wright J. M. Slow voltage-dependent changes in channel open-state probability underlie hysteresis of NMDA responses in Mg(2+)-free solutions. Neuron. 1992 Jan;8(1):181–187. doi: 10.1016/0896-6273(92)90119-x. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Romani A., Scarpa A. Regulation of cell magnesium. Arch Biochem Biophys. 1992 Oct;298(1):1–12. doi: 10.1016/0003-9861(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Clements J. D., Westbrook G. L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993 Oct 29;262(5134):754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Feltz A., Westbrook G. L. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995 Apr;15(4):2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands S. B., Barish M. E. Neuronal nicotinic acetylcholine receptor currents in phaeochromocytoma (PC12) cells: dual mechanisms of rectification. J Physiol. 1992 Feb;447:467–487. doi: 10.1113/jphysiol.1992.sp019012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M. M., Dani J. A. Structural basis for explaining open-channel blockade of the NMDA receptor. J Neurosci. 1995 Feb;15(2):1446–1454. doi: 10.1523/JNEUROSCI.15-02-01446.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


