Abstract
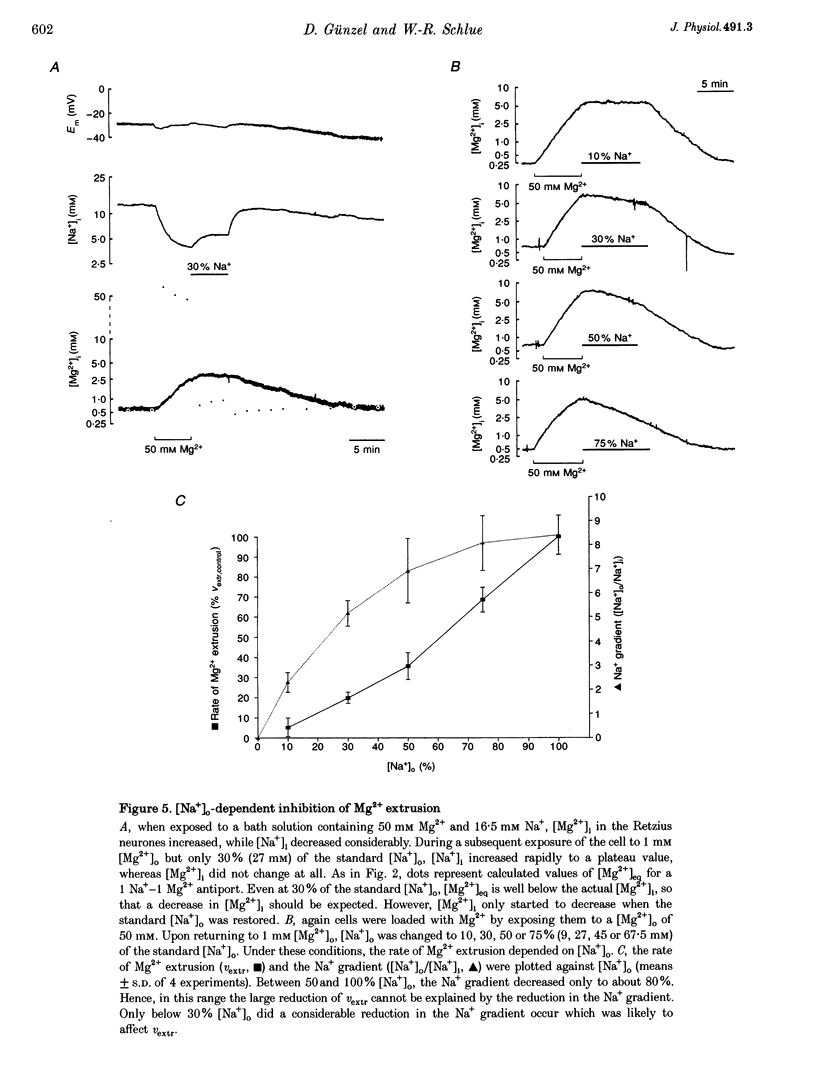
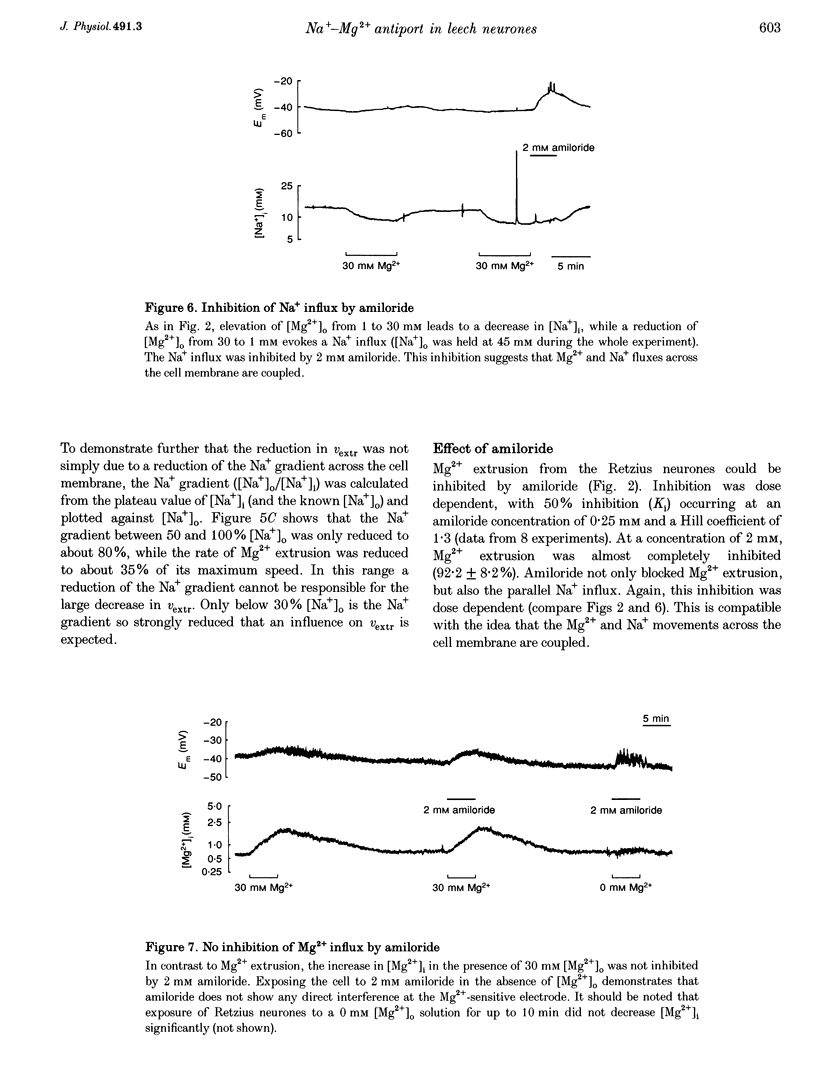
1. Intracellular free magnesium ([Mg2+]i) and sodium ([Na+]i) concentrations were measured in Retzius neurones of the leech Hirudo medicinalis using ion-sensitive microelectrodes. 2. The mean steady-state values for [Mg2+]i and [Na+]i were 0.46 mM (pMg, 3.34 +/- 0.23; range, 0.1-1.2 mM; n = 32) and 8.95 mM (pNa, 2.05 +/- 0.15; range, 5.1-15.5 mM, n = 21), respectively, at a mean membrane potential (Em) of -35.6 +/- 6.1 mV (n = 32). Thus, [Mg2+]i is far below the value calculated for a passive distribution (16.9 mM) but close to the equilibrium value calculated for a hypothetical 1 Na(+)-1 Mg2+ antiport (0.41 mM). 3. Simultaneous measurements of [Mg2+]i, [Na+]i and Em in Retzius neurones showed that an increase in the extracellular Mg2+ concentration ([Mg2+]o) resulted in an increase in [Mg2+]i, a parallel decrease in [Na+]i and a membrane depolarization, while a decrease in [Mg2+]o had opposite effects. These results are compatible with calculations based on a 1 Na(+)-1 Mg2+ antiport. 4. Na+ efflux at high [Mg2+]o still occurred when the Na(+)-K+ pump was inhibited by the application of ouabain or in K(+)-free solutions. This efflux was blocked by amiloride. 5. In the absence of extracellular Na+ ([Na+]o), no Mg2+ influx occurred. Mg2+ influx at high [Mg2+]o was even lower than in the presence of [Na+]o. Mg2+ efflux was blocked in the absence of [Na+]o. 6. The rate of Mg2+ extrusion was reduced by lowering [Na+]o, even if the Na+ gradient across the membrane remained almost unchanged. 7. Mg2+ efflux was blocked by amiloride (half-maximal effect at 0.25 mM amiloride; Hill coefficient, 1.3) but not by 5-(N-ethyl-N-isopropyl)-amiloride (EIPA). 8. No changes in intracellular Ca2+ and pH (pHi) could be detected when [Mg2+]o was varied between 1 and 30 mM. 9. Changing pHi by up to 0.4 pH units had no effect on [Mg2+]i. 10. The results suggest the presence of an electrogenic 1 Na(+)-1 Mg2+ antiport in leech Retzius neurones. This antiport can be reversed and is inhibited by low extracellular and/or intracellular Na+ and by amiloride.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Gamiño S. M., Rink T. J. Intracellular free magnesium in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1984 Sep;354:303–317. doi: 10.1113/jphysiol.1984.sp015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth J., Yassa R. Magnesium in mental illness. Compr Psychiatry. 1979 Sep-Oct;20(5):475–482. doi: 10.1016/0010-440x(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Buri A., Chen S., Fry C. H., Illner H., Kickenweiz E., McGuigan J. A., Noble D., Powell T., Twist V. W. The regulation of intracellular Mg2+ in guinea-pig heart, studied with Mg(2+)-selective microelectrodes and fluorochromes. Exp Physiol. 1993 Mar;78(2):221–233. doi: 10.1113/expphysiol.1993.sp003682. [DOI] [PubMed] [Google Scholar]
- Buri A., McGuigan J. A. Intracellular free magnesium and its regulation, studied in isolated ferret ventricular muscle with ion-selective microelectrodes. Exp Physiol. 1990 Nov;75(6):751–761. doi: 10.1113/expphysiol.1990.sp003457. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Eckert R., Schlue W. R. Changes in the intracellular free calcium concentration of Aplysia and leech neurones measured with calcium-sensitive microelectrodes. Can J Physiol Pharmacol. 1987 May;65(5):934–939. doi: 10.1139/y87-149. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. An ATP-dependent Na+/Mg2+ countertransport is the only mechanism for Mg extrusion in squid axons. Biochim Biophys Acta. 1988 Dec 22;946(2):424–428. doi: 10.1016/0005-2736(88)90418-x. [DOI] [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Fry C. H., Hall S. K., Blatter L. A., McGuigan J. A. Analysis and presentation of intracellular measurements obtained with ion-selective microelectrodes. Exp Physiol. 1990 Mar;75(2):187–198. doi: 10.1113/expphysiol.1990.sp003393. [DOI] [PubMed] [Google Scholar]
- Féray J. C., Garay R. An Na+-stimulated Mg2+-transport system in human red blood cells. Biochim Biophys Acta. 1986 Mar 27;856(1):76–84. doi: 10.1016/0005-2736(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Féray J. C., Garay R. Demonstration of a Na+: Mg2+ exchange in human red cells by its sensitivity to tricyclic antidepressant drugs. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):332–337. doi: 10.1007/BF00173409. [DOI] [PubMed] [Google Scholar]
- Günther T., Vormann J., Höllriegl V. Characterization of Na(+)-dependent Mg2+ efflux from Mg2(+)-loaded rat erythrocytes. Biochim Biophys Acta. 1990 Apr 30;1023(3):455–461. doi: 10.1016/0005-2736(90)90139-f. [DOI] [PubMed] [Google Scholar]
- Günzel D., Galler S. Intracellular free Mg2+ concentration in skeletal muscle fibres of frog and crayfish. Pflugers Arch. 1991 Jan;417(5):446–453. doi: 10.1007/BF00370938. [DOI] [PubMed] [Google Scholar]
- Hochstrate P., Schlue W. R. Ca2+ influx into leech glial cells and neurones caused by pharmacologically distinct glutamate receptors. Glia. 1994 Dec;12(4):268–280. doi: 10.1002/glia.440120404. [DOI] [PubMed] [Google Scholar]
- Lüdi H., Schatzmann H. J. Some properties of a system for sodium-dependent outward movement of magnesium from metabolizing human red blood cells. J Physiol. 1987 Sep;390:367–382. doi: 10.1113/jphysiol.1987.sp016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I., Lambert J. D., Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987 Mar;57(3):869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- Montes J. G., Sjodin R. A., Wu Y. K., Chen J. S., Yergey A. L., Vieira N. E. Regulation of potassium and magnesium effluxes by external magnesium in barnacle muscle fibres. Magnes Res. 1990 Dec;3(4):239–248. [PubMed] [Google Scholar]
- Russell J. M., Brodwick M. S. The interaction of intracellular Mg2+ and pH on Cl- fluxes associated with intracellular pH regulation in barnacle muscle fibers. J Gen Physiol. 1988 Apr;91(4):495–513. doi: 10.1085/jgp.91.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlue W. R. Effects of ouabain on intracellular ion activities of sensory neurons of the leech central nervous system. J Neurophysiol. 1991 Mar;65(3):736–746. doi: 10.1152/jn.1991.65.3.736. [DOI] [PubMed] [Google Scholar]
- Schlue W. R., Thomas R. C. A dual mechanism for intracellular pH regulation by leech neurones. J Physiol. 1985 Jul;364:327–338. doi: 10.1113/jphysiol.1985.sp015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. F., Hanck D. A. Mechanisms of extracellular divalent and trivalent cation block of the sodium current in canine cardiac Purkinje cells. J Physiol. 1992 Aug;454:299–320. doi: 10.1113/jphysiol.1992.sp019265. [DOI] [PMC free article] [PubMed] [Google Scholar]


