Abstract
Epstein-Barr viral (EBV) latency-associated promoters Qp, Cp, and LMP1p are crucial for the regulated expression of the EBNA and LMP transcripts in dependence of the latency type. By transient transfection and in vitro binding analyses, many promoter elements and transcription factors have previously been shown to be involved in the activities of these promoters. However, the latency promoters have only partially been examined at the nucleotide level in vivo. Therefore, we undertook a comprehensive analysis of in vivo protein binding and CpG methylation patterns at these promoters in five representative cell lines and correlated the results with the known in vitro binding data and activities of these promoters from previous transfection experiments. Promoter activity inversely correlated with the methylation state of promoters, although Qp was a remarkable exception. Novel protein binding data were obtained for all promoters. For Cp, binding correlated well with promoter activity; for LMP1p and Qp, binding patterns looked similar regardless of promoter activity.
Epstein-Barr virus (EBV) infection is the cause of infectious mononucleosis and is most closely associated with tumor diseases Burkitt's lymphoma (BL) and nasopharyngeal carcinoma. EBV infection of human B lymphocytes in vitro results in B-cell proliferation and transformation into continuously growing lymphoblastoid cell lines (LCL) (for a review, see reference 42). In latently infected cells, viral genomes are maintained as multiple circular episomal copies which are replicated once per cell cycle (2, 103). Several classes of latency have been described depending on the gene expression pattern (41, 77, 78). In strict type I latency, represented by BL cells, viral gene expression is restricted to the two RNA polymerase III-transcribed EBER RNA genes and the EBNA1 gene (78) that is transcribed from the Q promoter (Qp) (68). The EBNA1 protein is required for the maintenance of the viral plasmid in dividing cells (45, 58). In type III latency, in addition to the EBERs, EBNA-LP, -2, -3A, -3B, -3C, and -1 are expressed from the C promoter (Cp) (6), whereas LMP-1 and -2B are expressed from the bidirectional LMP1 promoter (46), and a larger splice variant of LMP-2, LMP-2A, is expressed from the TP1 promoter (36). Qp generally is supposed to be silent in type III latency (82, 105), although there is also a different view (93). Among the viral proteins expressed in latency type III, EBNA2 plays a central role in switching EBNA transcription from Wp to Cp (W to C switch) (102, 104) and in the establishment and maintenance of B-cell transformation (11, 28), as EBNA2 transcriptionally activates the expression of the six nuclear antigens from the C promoter (Cp) and the membrane proteins LMP-1 and -2B from the LMP1 promoter (LMP1p), LMP-2A from the TP1 promoter, and a number of cellular proteins associated with the LCL phenotype (1, 12, 18, 39, 44, 72, 76, 90, 95, 98, 99, 100, 101, 102, 104, 110, 111). A crucial mechanism involved in the silencing of Cp and LMP1p in type I latency has been shown to be methylation of CpG dinucleotides (3, 15, 35, 54, 60, 61, 70, 73, 74, 75, 84, 91, 94). In LCL, the EBV genome is mostly free of CpG methylation, whereas in BL cells, EBV genomes are highly methylated. An essential step in understanding the differences between latency types I and III is to elucidate the patterns of methylation and in vivo protein binding of the latency promoters of EBV at nucleotide resolution. Therefore, we decided to examine Qp, Cp, and LMP1p in cells of both latency types.
(The contributions of Daniel Salamon to this work were made in partial fulfillment of the requirements for a Ph.D. from Semmelweis University, Budapest, Hungary.)
MATERIALS AND METHODS
Cell lines and tissue culture.
LCL 721 is a B95-8-transformed LCL with type III phenotype (40, 52, 57). Rael (15, 43, 61) is a group I BL cell line. Mutu BLI-C1216 is a subclone of the BL line Mutu, representative of latency type I (27). Mutu BLIII-C199 is a subclone of the BL line Mutu, representative of latency type III (27). Raji cells express all the type III latency genes but use a thus far unknown promoter, other than Cp, for the EBNA transcripts (29, 96). All cells were maintained in suspension cultures of RPMI 1640 medium containing 10% fetal calf serum, 2 mM glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml at 5% CO2 and 37°C.
Electrophoretic mobility shift assay.
Preparation of nuclear extracts from Mutu I cells was essentially based on the standard of Dignam et al. (14). Nuclei were prepared using a combination and modification of two methods (8, 30) as already described (64). Complementary double-stranded DNA oligonucleotides (Metabion) containing a consensus binding site for CBF1, 5′-GGATCCGCCGTGGGAAAAAGTCGAC-3′, and a mutant binding site disabled for CBF1 binding, 5′-GGATCCGCCGTGTTAAAAAGTCGAC-3′, (51) were kinase labeled, annealed, and spin column purified for a gel shift probe. Gel retardation assays were performed as described (30, 64): 1 μg of crude nuclear protein was incubated with poly(dI-dC)as indicated, 1 ng of 32P-labeled probe, and a 50-fold excess of unlabeled competitor fragment in 25 μl of bandshift buffer (10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 80 mM KCl, 1 mM dithiothreitol, 1 mM EDTA, 12.5% glyceroi, 0.1% Triton X-100) for 20 min. Protein complexes were resolved by electrophoresis on native 4% polyacrylamide gels (29+1) in 6.7 mM Tris-HCl (pH 7.5)–3.3 mM sodium acetate (pH 7.0)–1 mM EDTA at 20 mA for several hours.
DNA sequences.
Oligonucleotides (Metabion, Martinsried, Germany) corresponding to EBV nucleotides (4) 10595 to 10614 and 11364 to 11342 were used for sequencing Cp, and others corresponding to nucleotides 62146 to 62165 and 62548 to 62524 were used for sequencing Qp. Both strands of the two promoters were sequenced from the genomic DNA of all five cell types on an ABI 377 DNA sequencing system using dye-labeled dideoxynucleoside triphosphates (ddNTPs). In the analyzed region of the C promoter (nucleotides 10615 to 11341), a few sequence polymorphisms were noted in Rael (GenBank accession number AJ297541) and in the Mutu subclones, whereas the sequences of LCL 721 and Raji were identical to the standard B95-8 sequence (4). The sequence of Cp between nucleotides 10615 and 11046 in the Mutu subclones is at GenBank numbers AJ000877 and AJ000878 (91), and the 3′ part between nucleotides 11047 and 11341 was identical with the standard sequence (4). The sequence of Qp between nucleotides 62166 and 62523 did not show any deviation from the B95-8 standard sequence in all cell types (4). Sequences of LMP1p have been described (91a).
Automated genomic sequencing of sodium bisulfite-treated DNA.
We used the method of Frommer et al. (22) and Clark et al. (10) adapted for an automated DNA sequencer (63). A total of 5 μg of genomic DNA in 50 μl of water was denatured by adding 5.5 μl of freshly prepared 3 M NaOH and incubating for 15 min at 37°C. Then 30.5 μl of freshly prepared 10 mM hydroquinone (Sigma), and 530 μl of 3.6 M sodium bisulfite, pH 5 (Sigma), were added to the denatured DNA, mixed gently, divided into five 0.5-ml PCR tubes, overlaid with paraffin oil, and cycled five times at 95°C for 3 min and 55°C for 57 min. After this treatment, the modified DNA was purified using a GeneClean kit (BIO 101) according to the manufacturer's instructions. Then the DNA was desulfonated by adding freshly prepared 3 M NaOH to a final concentration of 0.3 M and incubating the mixture for 15 min at 37°C. After desulfonation, the DNA was ethanol precipitated and dissolved in water. Then 100 ng of freshly modified DNA was used for PCR amplification with the strand-specific outer primer pairs (22) designed for the promoter regions (Table 1). The 50-μl PCR contained 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 2.5 mM MgCl2, 0.1% Triton X-100, 40 pmol each of the primers, 0.2 mM each of the four dNTPs, and 2 U of Taq polymerase (Promega). Then 3 μl of a 1:100 dilution from the first PCR was amplified in a second nested PCR using the primers listed in Table 1. One of the nested primers was biotin labeled, and the other carried 15 bases of the M13 universal primer at its 5′ end. The reaction mixture for the nested PCR was the same as for the first PCR except that the amount of inner primers was 10 pmol each. The product of the second PCR was bound to streptavidin-coated magnetic beads (Dynal), and the purified biotin-labeled strand was sequenced using the AutoRead DNA sequencing kit (Amersham Pharmacia Biotech) and a fluorescein-labeled M13 universal primer as described by Myöhänen et al. (63). The reaction products were separated on acrylamide gels using an automated DNA sequencer (Amersham Pharmacia Biotech). The degree of methylation was estimated as described earlier (63). The bisulfite conversion reaction was complete, since all cytosines outside CpG dinucleotides were converted to uracil and therefore sequenced as thymine instead of cytosine after PCR (see reference 80 and Takacs et al. [submitted] for examples).
TABLE 1.
Primers for methylation mappinga
| Primer type | No. | Sequence (nucleotides) |
|---|---|---|
| Outer primers for modified Qpb | 1 | GGTTAGTTTGATTAAGGGTGAGGT (62179–62202) |
| 2 | CCATTACCCCCAATACATTCC (62626–62606) | |
| Inner primers for modified Qpc | 3 | Univ-CTCCCAACTACCCAAAATACCA (62504–62483) |
| 4 | Biotin-GTTAGTTTGATTAAGGGTGAGGTTATAA (62180–62207) | |
| Outer primers for modified Cpd | 5 | GGGTTTAGGTTTTGTAGGGTAGA (10585–10607) |
| 6 | CCCTACRATAAAAACTCTAAAAATCTT (11392–11366) | |
| Inner primers for modified Cpe | 7 | Univ-GTTAGGTTGATAAGGGGATAAG (10610–10631) |
| 8 | Univ-TGAGAGGTTAGTGTTTTAAATATGT (10878–10902) | |
| 9 | Univ-GGATTATAGTTAATAAGAGAGTTTAAGA (11066–11093) | |
| 10 | Biotin-ATCCTTATCTCTATACCATCTAATCTA (11361–11335) |
Primer positions refer to the nucleotides of the B95-8 sequence (4). Univ indicates the M13 universal primer sequence GTAAAACGACGGCCA. Primers were purchased from Metabion (Martinsried, Germany). Each PCR was cycled 30 times at the temperatures and times indicated. Primers for LMP1p, PCR conditions, and the CpG methylation maps of LMP1p are described in detail by Takacs et al. (91a).
Cycled at 95°C for 40 s, 60°C for 40 s, and 72°C for 70 s.
Cycled at 95°C for 40 s, 58°C for 40 s, and 72°C for 60 s.
Cycled at 95°C for 40 s, 58°C for 40 s, and 72°C for 90 s.
Cycled at 95°C for 40 s, 52°C for 40 s, and 72°C for 90 s (7 to 10), 70 s (8 to 10), or 60 s (9 to 10).
DMS in vivo footprinting.
Genomic footprinting was performed essentially as described (65). For each footprint reaction, 107 exponentially growing cells were harvested, washed with phosphate-buffered saline (PBS), resuspended in 1 ml of PBS, and incubated at room temperature for 1 min with 5 μl of dimethyl sulfate (DMS). The reaction was stopped by the addition of 5 ml of DMS stop solution, containing 1% bovine serum albumin and 100 μM β-mercaptoethanol in PBS. Cells were washed once more in DMS stop solution and twice more with PBS. Finally, cells were resuspended in 1 ml of PBS, and genomic DNA was prepared. Footprinted DNAs were subjected to piperidine treatment (55). For visualization of footprints by ligation-mediated PCR (LM-PCR), 2 μg of sequenced or footprinted DNA was analyzed as described (26, 62) with modifications (65). The primers for LM-PCR are listed in Table 2. The first-strand primer extension reaction was done in 10 mM KCl–10 mM (NH4)2SO4–20 mM Tris-HCl–2 mM MgSO4–0.1% Triton X-100 (pH 8.8) at 25°C (Vent buffer; New England Biolabs), containing 0.3 pmol of primer i of each set, 240 μM each dNTP, and 1 U of Vent (exo-) DNA polymerase (New England Biolabs) for 5 min at 94°C, 30 min at 60°C, and 10 min at 72°C. For ligation of the common linker, the sample was transferred to ice, and 5 μl of PCR linker mix as in Mueller and Wold (62), 2 μl of ligation buffer (660 mM Tris-HCl, 50 mM MgCl2, 10 mM dithioerythritol, 10 mM ATP [pH 7.5] [20°C], Boehringer Mannheim), 1 μl of T4 DNA ligase (5 U/μl; Boehringer Mannheim), and 12 μl of water were added. After overnight incubation at 4°C, the DNA was ethanol precipitated, washed once with 75% ethanol, dried, and then resuspended in water. The PCR amplification was done in 100 μl of Vent buffer containing 10 pmol of each primer ii and the longer linker primer, 240 μM each dNTP, and 1 unit of Vent (exo-) DNA polymerase for 20 cycles using 1 min at 94°C, 1.5 min at 60°C, and 3 min at 72°C. For labeling, the sample was transferred to ice, 5 pmol of T4 kinase [γ-32P]ATP-labeled primer iii, 2.5 nmol of each dNTP, and 0.5 U of Vent (exo-) DNA polymerase in a volume of Vent buffer not exceeding 15 μl were added. Then the sample was heated to 94°C for 1.5 min, subjected to eight cycles of 2 min at 94°C, 2 min at 62°C, and 5 min at 72°C, and kept at 72°C for 5 more min. Samples were phenol-chloroform extracted, ethanol precipitated, ethanol washed, and resuspended in loading dye. One fifth of each sample was separated on a 5% sequencing gel, and the gels were dried and autoradiographed at room temperature with Kodak BioMax MR film.
TABLE 2.
Primers for LM-PCRa
| Promoter | Primer set | No. | Sequence (nucleotides) |
|---|---|---|---|
| Qp | A | i | GCTATAACGCAGGTCCTGTTCCGGG (62201–62225) |
| ii | GCGGTGGATAGAGAGGAGGGGGATC (62229–62253) | ||
| iii | GAGGGGACCACTAGGTCGCCGGAGG (62256–62280) | ||
| B | i | CCCCAAACATACACCGTGCGAAAAG (62548–62524) | |
| ii | CCGTGCGAAAAGAAGCACCCCCATC (62535–62511) | ||
| iii | CCGCCTCCCAGCTGCCCAAAATGCC (62508–62484) | ||
| Cp | A | i | GTCCCAATTAGAAACCCAAGCGCAG (10845–10869) |
| ii | CCCAAGCGCAGAAATTAGTTGAGAGG (10859–10884) | ||
| iii | AACMTGCACCCTAGGCCAGCCAGAG (10896–10920) | ||
| B | i | ACTTTGCGAGCCCTGCGTCTTGAG (11110–11087) | |
| ii | TATTGGCTATAATCCGTCGCTCCTCCC (11080–11054) | ||
| iii | CCGTCGCTCCTCCCAGATAAGGCGT (11067–11043) | ||
| C | i | CTCAAGACGCAGGGCTCGCAAAGT (11087–11110) | |
| ii | GTATAGTGGCCCCGTGGGACCTTAG (11109–11133) | ||
| iii | TTAGAGGTGGAGCAACGTCTAAAGTGG (11130–11156) | ||
| D | i | GGGCCTACATGGCCGCATGGTAAG (11418–11395) | |
| ii | GGTAAGAACCCTGCGATGAGGGCTC (11400–11376) | ||
| iii | GATGAGGGCTCTGGGGGTCTTCGGTG (11386–11361) | ||
| E | i | GTGCGTCGAGTGCTATCTTTGGAAC (10981–11005) | |
| ii | ACCTTGTTGGCGGGAGAAGGMATAAC (11019–11044) | ||
| iii | ACGCCTTATCTGGGAGGAGCGACGG (11043–11067) | ||
| LMP1p | A | i | CCCCTCTCAAGGTCGTGTTCCATCC (169452–169476) |
| i var | CCCCTCTCAAGGTCCAGGTCCATGC (169452–169476) | ||
| ii | TCAGGGCAGTGTGTCAGGAGCAAGG (169477–169501) | ||
| ii var | TCAGGGCAGTGTGTCAGGAGCCAGG (169477–169501) | ||
| iii | AGGCAGTTGAGGAAAGAAGGGGGCAG (169489–169524) | ||
| B | i | CTTAGCCCTCTTAGCCGCCTCACC (169966–169943) | |
| ii | TACGGTTACCCCACAGCCTTGCCTC (169933–169909) | ||
| ii var | TACGGTGAACCCACATCCTTGCCTC (169933–169909) | ||
| iii | GCCTCACCTGAACCCCCCTAAAGCAC (169913–169888) | ||
| iii var | GCCTCACCTGAACCCCCCTAAAACMC (169913–169888) | ||
| C | i | GCGCCTCTTTGTGCAGATTACACTG (169843–169819) | |
| ii | CCGCTTCCCACAACACTACGCACTC (169818–169794) | ||
| iii | CCTTCTGATTGCCGCACTGCCTTTCC (169791–169716) | ||
| D | i | GTACGGGYRCAGATTTCCCGAAAG (169621–169644) | |
| ii | GATTTCCCGAAAGCGGCGGTGTGTG (169632–169656) | ||
| iii | CGGCGGTGTGTGTGTGCATGTAAGCG (169645–169660) | ||
| E | i | AGAGGAGGAGAAGGAGAGCAAGG (169375–169397) | |
| ii | CCCCTCTCAAGGTCGTGTTCCATCC (169452–169476) | ||
| ii var | CCCCTCTCAAGGTCCAGGTCCATGC (169452–169476) | ||
| iii | TCAGGGCAGTGTGTCAGGAGCAAGG (169477–169501) | ||
| iii var | TCAGGGCAGTGTGTCAGGAGCCAGG (169477–169501) | ||
| F | i | CACACGCTTYCTACTTCCCCTTTYTAC (169696–169670) | |
| ii | CGCTTACATGCACACACACACCGCC (169670–169646) | ||
| iii | CACACACCGCCGCTTTCGGGAAATC (169656–169632) |
Primer positions refer to nucleotide numbers of the B95-8 sequence (4). Primers were purchased from Metabion (Martinsried, Germany). Variant primers or primers with wobble bases were used because of minor sequence deviations between EBV strains. With these primer sets, both strands of each promoter were visualized in their entire length, and several promoter parts were seen with more than one primer set.
RESULTS
Methylation patterns at CpG dinucleotides.
The methylation data solely reflected the status of tightly latent EBV circular genomes, but not the presence of linear genomes from a possible small amount of lytic replication as was tested by terminal repeat analysis through southern blotting (59, 91a) and for LCL 721 and Raji cells through Gardella gels, in addition (52). Early antigens or their coding mRNAs associated with productive EBV replication could not be detected either in the above-mentioned cell lines and clones (54, 57; J. Minarorits, unpublished data). In addition, specific segments of the EBV genome were found to be completely methylated in all five cell types (data not shown), another indication that there was no lytic cycle viral DNA in the cell lines examined. Cytosines between nucleotides 62264 and 62482 of the Qp region were completely unmethylated in all cell types (Fig. 1). The methylation data on Qp were in agreement with previous observations (84, 93), completed these observations for Mutu I, and extended them to the additional cell types LCL 721 and Mutu III. Previous methylation analyses of Cp (3, 54, 61, 74, 75, 84, 91, 94) could be largely confirmed and extended by our present work. Overally, Cp was nonmethylated in class III cell lines, but highly methylated in class I BL cell lines and Raji (Fig. 2). However, in Mutu I there was a methylation gap of about 100 bp of complete demethylation around the crucial CBF1 and CBF2 binding sites (16, 37, 74, 75), and a further adjoining gap of about 100 bp of partial demethylation (Fig. 2). For Mutu III there was a small contradiction to our earlier work (91), where CpG dinucleotides 10702 and 10799 were found to be highly methylated. Methylation at these two CpGs could not be confirmed anymore. The discrepancy was most likely due to a sequencing artifact in the earlier work. Methylation of LMP1p in the five cell lines has been recently examined by Takacs et al. (submitted). Overall, LMP1p was hypo- or nonmethylated in class III cell lines, as well as Raji, but highly methylated in class I BL cell lines (Fig. 3).
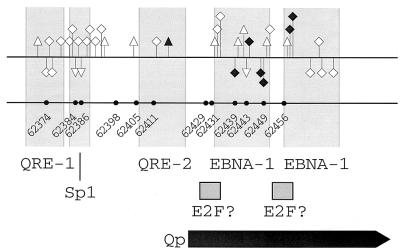
FIG. 1.
Summary of genomic footprinting and methylation patterns of Qp. Numbers and circles on the lower line indicate positions of cytosines within CpG dinucleotides and show that all CpG dinucleotides within Qp are totally unmethylated in all cell types. On the upper line, guanines protected from methylation by DMS are indicated by squares and enhanced reactivity to DMS is shown by triangles. Guanines that showed a different reactivity to DMS between cell types are indicated by solid symbols. The upper strand is shown above, and the lower strand is below the line. The positions of important cis regulatory elements are indicated by columns and boxes. The transcription initiation site of Qp is shown by a thick arrow.
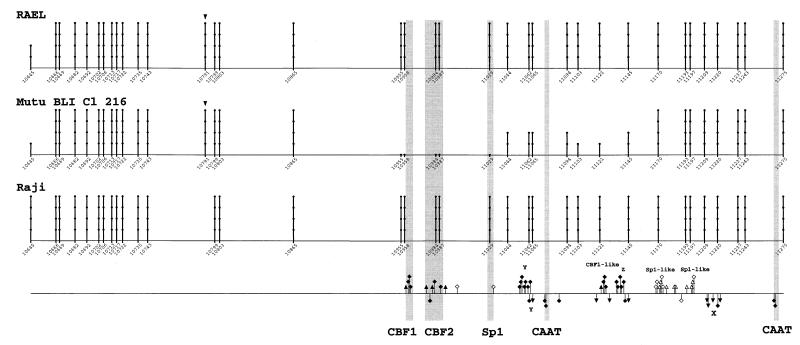
FIG. 2.
Summary of genomic footprinting and methylation patterns in the sequenced region of the BCR2 promoter (Cp). Numbers and lollipops indicate positions of cytosines within CpG dinucleotides, based on the prototype B95-8 sequence (4). Triangles above the lollipops mark additional target cytosines for DNA (cytosine-5) methyltransferase (5, 69) present in the cell lines studied. The degree of methylation of cytosines is indicated by the height of the lollipops as follows: spot only, 0%; one lollipop unit, 0 to 25%; two units, 25 to 50%; three units, 50 to 75%; four units, 75 to 100%. The bottom line shows a summary of genomic footprints for the upper (above the line) and lower (below the line) strand of Cp. Guanines protected from methylation by DMS are indicated by squares, and enhanced reactivity to DMS is shown by triangles. Guanines that showed a different reactivity to DMS between cell types are indicated by solid symbols. Novel footprints (X, Y, Z, CBF1-like, and Sp1-like) are indicated above and below the footprint marks. Faint columns represent already published relevant transcription factor binding sites in Cp.
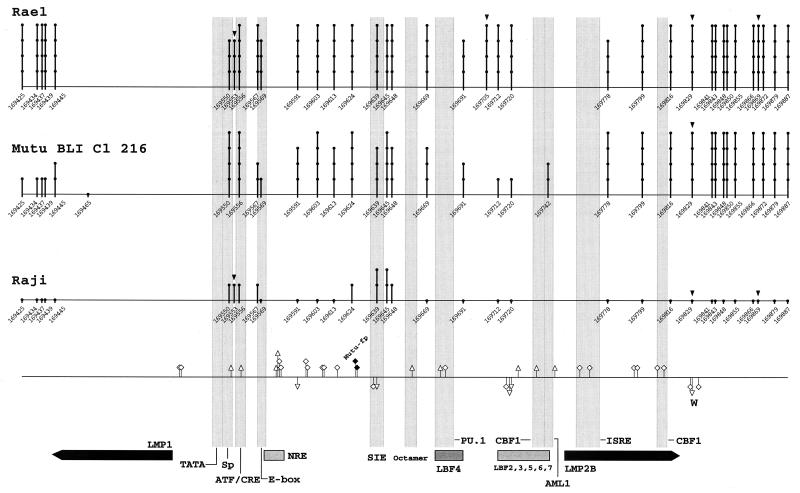
FIG. 3.
Summary of genomic footprinting and methylation patterns in the sequenced region of the LMP1 promoter. Numbers and lollipops indicate positions of cytosines within CpG dinucleotides, based on the prototype B95-8 sequence (4). Triangles above the lollipops mark additional target cytosines for DNA (cytosine-5) methyltransferase present in the cell lines studied. The degree of methylation of cytosines is indicated by the height of the as defined in the legend to Fig. 2. The bottom line shows a summary of genomic footprints for the upper (above the line) and lower (below the line) strand of LMP1p. Guanines protected from methylation by DMS are indicated by squares, and enhanced reactivity to DMS is shown by triangles. Some novel footprints (Mutu-fp with solid symbols and W) are indicated above and below the footprint marks. Faint columns represent important transcription factor binding sites in LMP1p. The transcription initiation sites of LMP1 and LMP2B are shown by thick arrows.
In vivo protein binding. (i) Q promoter.
The DMS footprinting was done on both strands of Qp (Fig. 4) according to standard methods (26, 62, 65). The pattern of guanines protected from methylation and nucleotides hypersensitive to methylation is summarized in Fig. 1. The footprint patterns on Qp from the five cell lines were generally identical, with four remarkable features. First, in Mutu I cells the EBNA1 binding sites were more weakly protected than in the other cell lines. Second, there was a hypersensitivity in the type III cell lines on the upper strand at guanine 62416 within QRE2, but not in the type I cells. Third, there was a strong protein-DNA interaction at a potential Sp1 binding site around nucleotides 62382 to 62394 (67). Fourth, footprints with a typical protection pattern indicative of E2F binding (112), at two previously characterized unconventional E2F sites, interspersed with the EBNA1-sites and the transcriptional start site (13, 89), were not found.
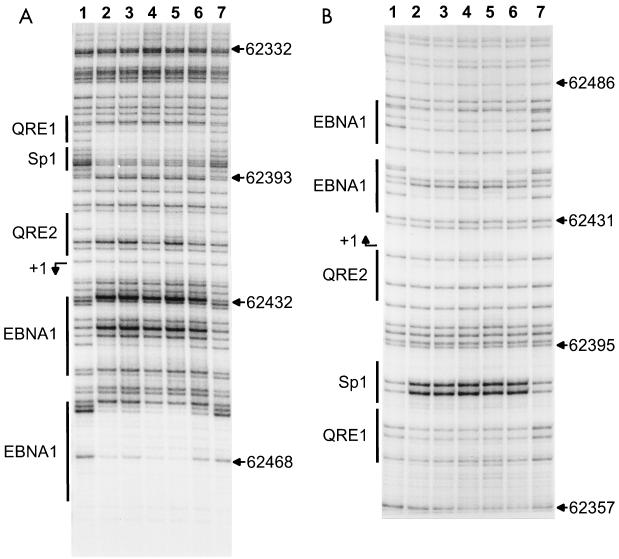
FIG. 4.
Genomic footprint analyses of Qp. (A) Upper strand, (B) Lower strand. Lane 1, G track from LCL 721 DNA; lanes 2 to 6, footprints. Lane 2, LCL 721 cells; lane 3, Mutu III cells; lane 4, Rael cells; lane 5, Raji cells; lane 6, Mutu I cells; lane 7, G track from Mutu I DNA. At the left of each panel, the locations of in vivo footprints and previously described in vitro binding sites are indicated by vertical bars; at the right of each panel, nucleotide numbers are given according to the EBV sequence of Baer et al. (4).
(ii) C promoter.
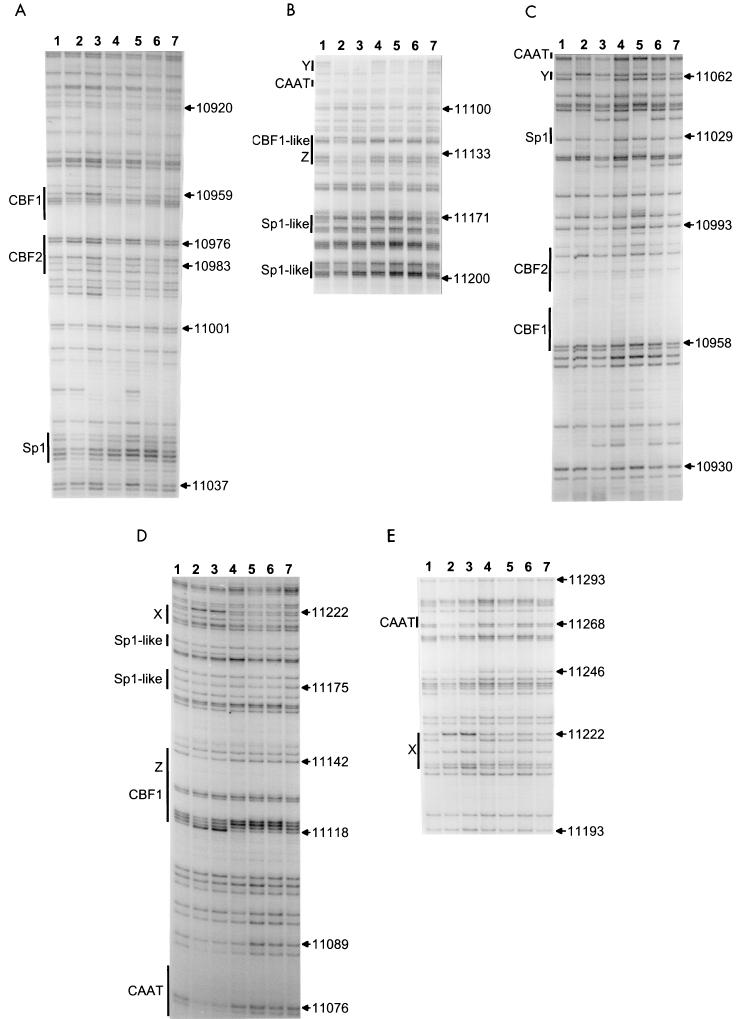
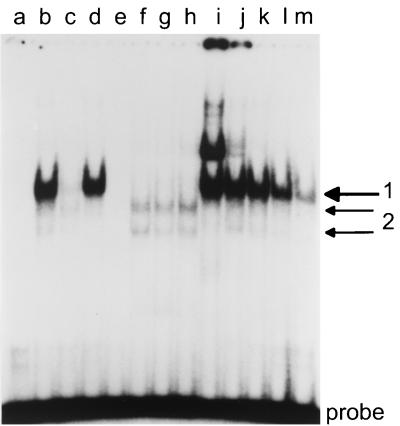
Previous in vitro binding and reporter gene experiments have charted a CBF1 site, a CBF2 site, and two CCAAT boxes as transcriptional elements of Cp and identified CBF1/RBP-Jκ and AUF1 as the respective binding proteins for the CBF1 and CBF2 sites (23, 25, 37, 47, 50, 71, 75). These binding sites and an additional Sp1 site have been shown to be highly conserved between EBV and two related lymphocryptoviruses of monkeys (24). The promoter area examined here is shown in Fig. 5. The pattern of guanines protected from methylation and nucleotides hypersensitive to methylation is summarized together with the methylation data in Fig. 2. There were two Sp1-like sequences around nucleotides 11176 and 11197 that were protected in all cell lines despite methylation (31). The Sp1 site at 11029 showed slight signs of protein-DNA interaction in all cells. Further, we found a series of sites protected only in 721 and Mutu III cells, but not protected in type I and Raji cells. The two CAAT boxes and several novel protections belonged to this category. The CAAT box at nucleotide 11075 was strongly protected, and the CAAT box at nucleotide 11268 was strongly protected in LCL 721 cells and weakly in Mutu III cells. A novel interaction, called X, was found around nucleotide 11222. Another novel interaction, called Y, carrying no familiar consensus sequence for transcription factor binding, was found around nucleotide 11062. An extended interaction was found between 11120 and 11140, with a methylation interference pattern characteristic of CBF1 binding (56) in the 5′ part and a protection, called Z, in the 3′ part. Binding at the CBF2 site in LCL 721 and Mutu III cells was weak at best, since there were slight signs of protein-DNA interaction only (Fig. 5). A characteristic protein-DNA interaction was found at the previously described CBF1 site at nucleotide 10959. Since the CBF1 site was unmethylated but the protection pattern was not typical in Mutu I, we performed electrophoretic mobility shift experiments. The gel shifts showed that a CBF1-like binding activity was present in Mutu I and was able to bind to its consensus site in a sequence-specific manner (Fig. 6).
FIG. 5.
Genomic footprint analyses of Cp. (A and B) Upper strand. (C, D, and E) Lower strand. Lane 1, G track from LCL 721 DNA; lanes 2 to 6, footprint lanes. Lane 2, LCL 721 cells; lane 3, Mutu III cells; lane 4, Rael cells; lane 5, Raji cells; lane 6, Mutu I cells; lane 7; G track from Mutu I DNA. At the left of each panel, the locations of in vivo footprints, of previously described in vitro footprints, and of consensus elements are indicated by vertical bars; at the right of each panel, nucleotide numbers are given according to the EBV sequence of Baer et al. (4).
FIG. 6.
Electrophoretic mobility shift assay: binding of nuclear proteins from Mutu I cells to the CBF1 site and to a mutant control oligonucleotide. Labeled double-stranded oligonucleotides containing a CBF1 consensus site and a mutant site disabled for CBF1 binding were incubated with 1 μg of crude nuclear extract from Mutu I cells with the oligonucleotides, amounts of poly(dI-dC), and unlabeled competitor oligonucleotides as indicated. The resulting protein-DNA complexes were separated in a 4% polyacrylamide gel. Lanes a to h and k, 1 μg of poly(dI-dC) added; lanes a to d and i to m, labeled CBF1 oligonucleotide as a probe; lanes e to h mutant oligonucleotide labeled as a probe. Lane 2, no protein added; lane b, nuclear extract; lane c, shift competed with a 50-fold excess of unlabeled CBF1 oligonucleotide; lane d, competition with a 50-fold excess of unlabeled mutant oligonucleotide; lane e, no protein added; lane f, nuclear extract; lane g, competition with a 50-fold excess of unlabeled CBF1 oligonucleotide; lane h, competition with a 50-fold excess of unlabeled mutant oligonucleotide; lane i, 0.1 μg of poly(dI-dC) added; lane j, 0.5 μg of poly(dI-dC); lane k, 2 μg of poly(dI-dC); lane m, 5 μg of poly(dI-dC) added.
(iii) LMP1 promoter.
Many transcriptional elements of LMP1p have been characterized so far by in vitro binding and reporter gene experiments. Among these elements were binding sites for CBF1 (38, 51); PU.1, also called Spi-1, and Spi-B (38, 47, 48, 85, 108); AML1, also called LBF1, and several LMP1p binding proteins, named LBF-2 to -7 (38); negative regulatory element NRE (18); the E box carrying a USF binding site (88); a cis-inducible element (SIE) (86, 87); and an ATF/CRE that, depending on the distinct proteins binding, was able to activate LMP1p both independently and dependent on EBNA2 (20, 87, 88). The relevant footprinted promoter area is shown in Fig. 7. The pattern of guanines protected from methylation and nucleotides hypersensitive to methylation is summarized together with the methylation data in Fig. 3. Footprints were generally identical for all cell types. Signs for protein-DNA interactions, mostly of low or intermediate strength, were found on either one or both strands of the PU.1, AML1, LBF-3, 5, 6, 7, Oct, ISRE, SIE, NRE, and Sp1-like site, the two CBF1 sites, and the ATF/CRE site. The PU.1/LBF4 binding activity carried the typical methylation interference pattern described for these factors in vitro (38). The CBF1 footprints did not carry the typical methylation interference pattern (56) that was found in type III cells except Raji on Cp. In addition to protein-DNA interactions at already charted elements, we found a footprint at nucleotides 169520 and 169521 around the initiation site of the LMP1 transcript, a hypersensitive site at nucleotide 169591, a footprint at 169596 and 169597, a footprint at 169606 and 169607, a footprint at 169615, and a strong footprint, called W, at nucleotide 169833 for all cell types examined. In addition, we found a footprint, Mutu-fp, specific for both Mutu clones at nucleotides 169626 and 169627. These binding factors await further identification. However, there were no differences in the in vivo binding pattern, with the possible exception of the ATF/CRE-Spl locus. At this locus we found slight differences in the reactivity to DMS between cells.
FIG. 7.
Genomic footprint analyses of LMP1p. (A, B, C, D, and E) Upper strand. (F, G, and H) Lower strand. (A) Lane 1, G track from LCL 721 DNA; lane 2, footprint from LCL 721 cells; lane 3, footprint from Mutu III cells; lane 4, footprint from Mutu I cells; lane 5, G track from Rael DNA; lane 6, footprint from Rael cells; lane 7, G track from Raji DNA; lane 8, footprint from Raji cells. (B and C) Lane 1, G track from LCL 721 DNA; lanes 2 to 6, footprints. Lane 2, LCL 721 cells; lane 3, Mutu III cells; lane 4, Rael cells; lane 5, Raji cells; lane 6, Mutu I cells; lane 7, G track from Raji cells. (B) Protection of guanines against methylation by DMS indicated by lollipops. (D and E) Lane 1, G track from LCL 721 DNA, lanes 2 to 6, footprints; lane 2, LCL 721 cells; lane 3, Mutu III cells; lane 4, Rael cells; lane 5, Raji cells; lane 6, Mutu I cells; lane 7, G track from Mutu I cells; lane 8, G track from Rael cells. (F) Lane 1, G track from LCL 721 DNA; lanes 2 to 6, footprints. Lane 2, LCL 721 cells; lane 3, Mutu III cells; lane 4, Rael cells; lane 5, Raji cells; lane 6, Mutu I cells; lane 7, G track from Mutu I DNA; lane 8, G track from Raji DNA. (G and H) Lane 1, G track from LCL 721 DNA; lane 2, footprint from LCL 721 cells; lane 3, footprint from Mutu III cells; lane 4, G track from Mutu I DNA; lane 5, footprint from Mutu I cells; lane 6, G track from Rael DNA; lane 7, footprint from Rael cells; lane 8, G track from Raji DNA; lane 9, footprint from Raji cells. At the left of each panel, the locations of in vivo footprints, of previously described in vitro footprints, and of consensus elements are indicated by vertical bars, and at the right of each panel, nucleotide numbers are given according to the EBV sequence of Baer et al. (4).
DISCUSSION
Although under some conditions Mutu I may drift to type III latency, Mutu BLI-C1216 of this study represents a type I cell, because of its phenotype and EBNA-2 protein could not be found by Western blotting (data not shown). Still, we cannot entirely rule out the possibility that an extremely small proportion of Mutu I cells were drifting towards type III latency. Because of this and because of the limited number of cell types in this study, final conclusions regarding the two latency types may only be drawn after the examination of a larger panel of cell lines and subclones.
Qp behaves like a bacterial promoter.
Qp was unmethylated and extensively protein protected in all cell types, regardless of the activity of the promoter (Fig. 1 and 4). The protein binding pattern was generally in congruence with the in vitro (9, 66, 67, 83, 89, 106) and in vivo (33) data described earlier. It is clear now that the Sp1-like sequence just downstream of QRE1 is strongly protein bound. This site has been discussed as a potential unconventional E2F site, but has been shown not to compete for the in vitro binding of E2F-like proteins (79). The overall protection pattern was identical in the five cell types, with minor exceptions: at the QRE2 element there was a hypersensitivity indicative of closer protein binding at QRE2 for the silent promoter state in LCL 721, Mutu III, and Raji cells (Fig. 4). Since Qp is unmethylated and heavily protein protected, the QRE2-bound protein may be key to the silencing of Qp in type III latency. Candidate factors for this binding activity are IRF-7, a Qp-repressive factor described previously (66, 106), and IRF-2, although there are contrary views on the repressive nature of IRF-2 (83, 107). The EBNA1 binding sites were protected in all cells, in agreement with Hsieh et al. (33), who have already demonstrated the same in vivo EBNA1 site protection in Qp for Raji cells. The weaker EBNA1 binding in Mutu 1 may be interpreted in terms of promoter activity in type I cells and a repressive function for EBNA1. However, in Rael, where Qp is active, the EBNA protection is as strong as in the type III cells. A clear protection pattern indicating E2F binding (112) could not be found at the sites previously described as in vitro E2F binding sites (79, 89). Therefore, the repressive role for EBNA1 (81) and the activating role for E2F in Qp transcription that have been postulated (79, 89) may have to be modified. The constant strong binding of EBNA1 together with Sp1 may cause the constitutive hypomethylation of Qp (7, 32, 49, 53, 80). In summary, Qp activity is likely to be regulated in a comparably simple way, as in bacterial promoters, by the binding or not of a few key transcription factors and a repressor.
Cp is regulated by methylation and protein binding.
Cp was unmethylated in the activity promoter state, but methylated in the inactive state (Fig. 2). The inverse correlation between methylation status and promoter activity was best in the promoter-proximal part, where Cp was completely methylated in the cell types not using Cp (Fig. 2). Therefore, extented alterations in overall CpG methylation seem to be more important than methylation of particular CpG dinucleotides in Cp (75). In agreement with earlier observations (16, 24, 70, 74), additional protein determinants of Cp activity besides CBF1 and CBF2 may play a role (Fig. 2 and 5). In addition to a couple of footprints at Sp1-like sequences that were common to all cells, there were several prominent footprints only found at active Cp that were completely lacking from inactive Cp. Differential footprinting was found at two sites for CBF1 and two CAAT boxes and three sites preliminarily named X, Y, and Z. The identity of these presumably activating transcription factors has yet to be established. CBF1 site protection patterns of Cp were remarkable because they were not identical in all cells, but correlated with promoter activity. Even in Mutu I cells, where the CBF1 binding sites are hypo- or unmethylated, there is the protection pattern of inactive Cp. This pattern is different from the typical CBF1 binding pattern, as demonstrated by methylation interference analysis (56). The difference is not due to the lack of CBF1 binding activity in Mutu I (Fig. 6). Previously published models (73, 109) supposed that the repressive factor CBF1 is constitutively bound to its binding sites independent of the CpG methylation status of the binding site. In that model, promoter activation occurs when activating transcription factor EBNA2 binds to already promoter-bound repressor CBF1, thereby covering the transcriptionally repressive domain of CBF1 (34). Therefore, the nontypical CBF1 site pattern of inactive Cp may be due to the activity of an additional negative regulator, like KyoT2 (92), or the typical pattern (56) was caused by CBF1 and additional protein. Alternatively, we might assume that CBF1 is not at all bound at the inactive Cp. The major differences in protein binding and methylation may be a hint for a restructuring between the active and inactive Cp (17).
LMP1p is regulated by methylation.
LMP1p has recently been examined in the five cell lines by Takacs et al. (submitted). LMP1p was hypo- or unmethylated in the active promoter state, but methylated in the inactive state. The detailed methylation maps (Fig. 3) were adapted from Takacs et al. (91a). Falk et al. (21) described comparable levels of methylation for both Mutu I and Mutu III cells. Our results for Mutu, however, were quite different. We found zero methylation in Mutu III and a high overall methylation in Mutu I cells. However, methylation in Mutu I was medium or less from nucleotides 169425 to 169465, at 169567 and 169569, and from 169691 to 169742. The discrepancy between the data of Falk et al. (21) and our data may be due to the use of a different clone of Mutu I and a different passage of clone BLIII-C199 for Mutu III. However, our methylation data on LMP1p fit to the promoter activities in the subclones of Mutu. In the previous literature on LMP1p, many binding sites have been characterized (19, 20, 38, 47, 48, 51, 56, 85, 87, 88, 97). Almost all previously characterized in vitro binding sites also carry signs of protein binding in vivo. However, this in vivo protection is visible regardless of LMP1p activity (Fig. 3 and 7). Therefore, since promoter protection patterns are identical in all cell types, the CpG methylation status seems to be the major determinant of promoter activity for LMP1p. Promoter activation is likely to be regulated by CpG demethylation and by alterations at the protein level that are transparent to genomic footprinting. Alternatively, there might be differences in promoter binding at other relevant promoter areas that we did not locate. Another possibility is that binding differences, especially at the ATF/CRE-Sp1 locus, are invisible to in vivo footprinting by DMS alone. These differences were very weak at best and were difficult to evaluate because of several sequence polymorphisms in this part of LMP1p. In vivo differences might be seen with the use of additional reagents for footprinting. We conclude that binding of all the factors involved in promoter activation at their respective binding sites is not sufficient to activate or repress LMP1p in vivo.
In summary, the contributions of CpG methylation and protein binding to promoter activity are in each case different for the three EBV latency-associated promoters Qp, Cp, and LMP1p. It would come as no surprise if the promoters for TP1, W, and the EBER RNAs also presented different pictures.
ACKNOWLEDGMENTS
We thank Ursula König and Fritz Schwarzmann for help with Western blotting; Cecilia Kerekgyarto-Zalka for excellent technical assistance; Marion Venus for excellent art work; Holger Melzl for expert help in sequencing PCR-amplified promoter fragments; Gergely Kadar for expert help in preparing some of the figures; György Berencsi (Division of Virology, National Center for Epidemiology), Zoltan Bori (Molecular Genetics Research Group, Hungarian Academy for Sciences, Budapest), and Peter Lakatos (First Department of Internal Medicine, Semmelweis University, Budapest) for kindly providing laboratory space for some of the experiments; and Istvan Földes for critically reading the manuscript.
The support of Ph.D. program 9 (led by Anna Kadar and Andras Falus) of Semmelweis University, Budapest, Hungary, is kindly acknowledged. Maria Takacs was a recipient of a Bolyai Fellowship awarded by the Hungarian Academy of Sciences. This work was in part supported by grant T 029710 of the National Science Foundation (OTKA), Hungary, and grant Wo 227/7 of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Abbott S D, Rowe M, Cadwallader K, Ricksten A, Gordon J, Wang F, Rymo L, Rickinson A B. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J Virol. 1990;64:2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams A. Replication of latent Epstein-Barr virus genomes in Raji cells. J Virol. 1987;61:1743–1746. doi: 10.1128/jvi.61.5.1743-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altiok E, Minarovits J, Hu L-F, Contreras-Brodin B, Klein G, Ernberg I. Host-cell-phenotype-dependent control of the BCR2/BWR1 promoter complex regulates the expression of Epstein-Barr virus nuclear antigens 2–6. Proc Natl Acad Sci USA. 1992;89:905–909. doi: 10.1073/pnas.89.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 5.Bird A. DNA methylation de novo. Science. 1999;286:2287–2288. doi: 10.1126/science.286.5448.2287. [DOI] [PubMed] [Google Scholar]
- 6.Bodescot M, Perricaudet M, Farrell P J. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol. 1987;61:3424–3430. doi: 10.1128/jvi.61.11.3424-3430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandeis M, Frank D, Keshtet I, Siegfried Z, Mendelsohn M, Nemes A, Temper V, Razin A, Cedar H. Spl elements protect a CpG island from de novo methylation. Nature. 1994;371:435–438. doi: 10.1038/371435a0. [DOI] [PubMed] [Google Scholar]
- 8.Bunce C M, Thick J A, Lord J M, Mills D, Brown G. A rapid procedure for isolating hemopoietic cell nuclei. Anal Biochem. 1988;175:67–73. doi: 10.1016/0003-2697(88)90362-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Lee J M, Wang Y, Huang D P, Ambinder R F, Hayward S D. The Epstein-Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity. Proc Natl Acad Sci USA. 1999;96:9339–9344. doi: 10.1073/pnas.96.16.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark S J, Harrison J, Paul C L, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen J I, Wang F, Mannick J, Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci USA. 1989;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordier M, Calender A, Billaud M, Zimber U, Rousselet G, Pavlish O, Banchereau J, Tursz T, Bornkamm G, Lenoir G M. Stable transfection of Epstein-Barr virus (EBV) nuclear antigen 2 in lymphoma cells containing the EBV P3HR1 genome induces expression of B-cell activation molecules CD21 and CD23. J Virol. 1990;64:1002–1013. doi: 10.1128/jvi.64.3.1002-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport M G, Pagano J S. Expression of EBNA-1 mRNA is regulated by cell cycle during Epstein-Barr virus type I latency. J Virol. 1999;73:3154–3161. doi: 10.1128/jvi.73.4.3154-3161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian cell nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernberg I, Falk K, Minarovits J, Busson P, Tursz T, Masucci M G, Klein G. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J Gen Virol. 1989;70:2989–3002. doi: 10.1099/0022-1317-70-11-2989. [DOI] [PubMed] [Google Scholar]
- 16.Evans T J, Farrell P J, Swaminathan S. Molecular genetic analysis of Epstein-Barr virus Cp promoter function. J Virol. 1996;70:1695–1705. doi: 10.1128/jvi.70.3.1695-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans T, Jacquemin M G, Farrell P J. Efficient EBV superinfection of group I Burkitt's lymphoma cells distinguishes requirements for expression of the Cp viral promoter and can activate the EBV productive cycle. Virology. 1995;206:866–877. doi: 10.1006/viro.1995.1009. [DOI] [PubMed] [Google Scholar]
- 18.Fahraeus R, Jansson A, Ricksten A, Sjöblom A, Rymo L. Epstein-Barr virus encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc Natl Acad Sci USA. 1990;87:7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahraeus R, Jansson A, Sjöblom A, Nilsson T, Klein G, Rymo L. Cell phenotype-dependent control of Epstein-Barr virus latent membrane protein 1 gene regulatory sequences. Virology. 1993;195:71–80. doi: 10.1006/viro.1993.1347. [DOI] [PubMed] [Google Scholar]
- 20.Fahraeus R, Palmqvist L, Nerdstedt A, Farzad S, Rymo L, Lain S. Response to cAMP levels of the Epstein-Barr virus EBNA2-inducible LMP1 oncogene and EBNA2 inhibition of a PP1-like activity. EMBO J. 1994;13:6041–6051. doi: 10.1002/j.1460-2075.1994.tb06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk K I, Szekely L, Aleman A, Ernberg I. Specific methylation patterns in two control regions of Epstein-Barr virus latency: the LMP-1-coding upstream regulatory region and an origin of DNA replication (oriP) J Virol. 1998;72:2969–2974. doi: 10.1128/jvi.72.4.2969-2974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frommer M, McDonald L E, Millar D S, Collis C M, Watt F, Grigg G W, Molloy P L, Paul C L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuentes-Panana E M, Ling P D. Characterization of the CBF2 binding site within the Epstein-Barr virus latency C promoter and its role in modulating EBNA2-mediated transactivation. J Virol. 1998;72:693–700. doi: 10.1128/jvi.72.1.693-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuentes-Panana E M, Swaminathan S, Ling P D. Transcription activation signals found in the Epstein-Barr virus (EBV) latency C promoter are conserved in the latency C promoter sequences from baboon and rhesus monkey EBV-like lymphocryptoviruses (Cercopithicine herpesviruses 12 and 15) J Virol. 1999;73:826–833. doi: 10.1128/jvi.73.1.826-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuentes-Panana E M, Peng R, Brewer G, Tan J, Ling P D. Regulation of the Epstein-Barr virus C promoter by AUF1 and the cyclic AMP/protein kinase A signaling pathway. J Virol. 2000;74:8166–8175. doi: 10.1128/jvi.74.17.8166-8175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrity P A, Wold B. Effects of different DNA polymerases in ligation-mediated PCR: enhanced genomic sequencing and in vivo footprinting. Proc Natl Acad Sci USA. 1992;89:1021–1025. doi: 10.1073/pnas.89.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 28.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 29.Hatfull G, Bankier A T, Barrell B G, Farrell P J. Sequence analysis of Raji Epstein-Barr virus DNA. Virology. 1988;164:334–340. doi: 10.1016/0042-6822(88)90546-6. [DOI] [PubMed] [Google Scholar]
- 30.Hennighausen L, Lubon H. Interaction of protein and DNA in vitro. Methods Enzymol. 1987;152:721–735. doi: 10.1016/0076-6879(87)52076-6. [DOI] [PubMed] [Google Scholar]
- 31.Holler M, Westin G, Jiricny J, Schaffner W. Spl transcription factor binds DNA and activates transcription even when the binding site is CpG methylated. Genes Dev. 1988;2:1127–1135. doi: 10.1101/gad.2.9.1127. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh C-L. Evidence that protein binding specifies sites of DNA demethylation. Mol Cell Biol. 1999;19:46–56. doi: 10.1128/mcb.19.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh D-J, Camiolo S M, Yates J L. Constitutive binding of EBNA1 protein to the Epstein-Barr virus replication origin, oriP, with distortion of DNA structure during latent infection. EMBO J. 1993;12:4933–4944. doi: 10.1002/j.1460-2075.1993.tb06187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh J J-D, Hayward S D. Masking of the CBF1/RBPJK transcriptional repression domain by Epstein-Barr virus EBNA2. Science. 1995;268:560–563. doi: 10.1126/science.7725102. [DOI] [PubMed] [Google Scholar]
- 35.Hu L F, Minarovits J, Cao S-L, Contreras-Salazar B, Rymo L, Falk K, Klein G, Ernberg I. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5′-flanking region. J Virol. 1991;65:1558–1567. doi: 10.1128/jvi.65.3.1558-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson G S, Farrell P J, Barrell B G. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95–8. J Virol. 1985;53:528–535. doi: 10.1128/jvi.53.2.528-535.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin X W, Speck S H. Identification of critical cis elements involved in mediating Epstein-Barr virus nuclear antigen 2-dependent activity of an enhancer located upstream of the viral BamHI C promoter. J Virol. 1992;66:2846–2852. doi: 10.1128/jvi.66.5.2846-2852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman S R. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by Jκ and PU. 1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavathas P, Bach F H, DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase 1 alleles in lymphoblastoid cells. Proc Natl Acad Sci USA. 1980;77:4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaye K M, Izumi K M, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr B M, Lear A L, Rowe M, Croom-Carter D, Young L S, Rookes S M, Gallimore P H, Rickinson A B. Three transcriptionally distinct forms of Epstein-Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology. 1992;187:189–201. doi: 10.1016/0042-6822(92)90307-b. [DOI] [PubMed] [Google Scholar]
- 42.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 43.Klein G, Dombos L, Gothosakar M. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB virus. Int J Cancer. 1972;10:44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- 44.Knutson J C. The level of c-fgr RNA is increased by EBNA-2, an Epstein-Barr virus gene required for B-cell immortalization. J Virol. 1990;64:2530–2536. doi: 10.1128/jvi.64.6.2530-2536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krysan P J, Haase S B, Calos M P. Isolation of human sequences that replicate autonomously in human cells. Mol Cell Biol. 1989;9:1026–1033. doi: 10.1128/mcb.9.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laux G, Economou A, Farrell P J. The terminal protein gene 2 of Epstein-Barr virus is transcribed from a bidirectional latent promoter region. J Gen Virol. 1989;70:3079–3084. doi: 10.1099/0022-1317-70-11-3079. [DOI] [PubMed] [Google Scholar]
- 47.Laux G, Adam B, Strobl L J, Moreau-Gachelin F. The Spi-1/PU.1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-Jκ interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. EMBO J. 1994;13:5624–5632. doi: 10.1002/j.1460-2075.1994.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laux G, Dugrillon F, Eckert C, Adam B, Zimber-Strobl U, Bornkamm G W. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane protein and terminal protein 2 genes. J Virol. 1994;68:6947–6958. doi: 10.1128/jvi.68.11.6947-6958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin I G, Tomzynski T J, Ou Q, Hsieh C L. Modulation of DNA binding protein affinity directly affects target site demethylation. Mol Cell Biol. 2000;20:2343–2349. doi: 10.1128/mcb.20.7.2343-2349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ling P D, Rawlins D R, Hayward S D. The Epstein-Barr virus immortalizing protein EBNA-2 is targeted to DNA by a cellular enhancer-binding protein. Proc Natl Acad Sci USA. 1993;90:9237–9241. doi: 10.1073/pnas.90.20.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling P D, Hsieh J J-D, Ruf I K, Rawlins D R, Hayward S D. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J Virol. 1994;68:5375–5383. doi: 10.1128/jvi.68.9.5375-5383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little R D, Schildkraut C L. Initiation of latent DNA replication in the Epstein-Barr virus genome can occur at sites other than the genetically defined origin. Mol Cell Biol. 1995;15:2893–2903. doi: 10.1128/mcb.15.5.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macleod D, Charlton J, Mullins J, Bird A P. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 1994;8:2282–2292. doi: 10.1101/gad.8.19.2282. [DOI] [PubMed] [Google Scholar]
- 54.Masucci M G, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, Klein G. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma line Rael. J Virol. 1989;63:3135–3141. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 56.Meitinger C, Strobl L J, Marschall G, Bornkamm G W, Zimber-Strobl U. Crucial sequences within the Epstein-Barr virus TP1 promoter for EBNA2-mediated transactivation and interaction of EBNA2 with its responsive element. J Virol. 1994;68:7497–7506. doi: 10.1128/jvi.68.11.7497-7506.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metzenberg S. Levels of Epstein-Barr virus DNA in lymphoblastoid cell lines are correlated with frequencies of spontaneous lytic growth but not with levels of expression of EBNA-1, EBNA-2, or latent membrane protein. J Virol. 1990;64:437–444. doi: 10.1128/jvi.64.1.437-444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Middleton T, Sugden B. Retention of plasmid DNA in mammalian cells is enhanced by binding of the Epstein-Barr virus replication protein EBNA1. J Virol. 1994;68:4067–4071. doi: 10.1128/jvi.68.6.4067-4071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minarovits J, Hu L-F, Imai S, Harabuchi Y, Kataura A, Minarovits-Kormuta S, Osato T, Klein G. Clonality, expression and methylation patterns of the Epstein-Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol. 1994;75:77–84. doi: 10.1099/0022-1317-75-1-77. [DOI] [PubMed] [Google Scholar]
- 60.Minarovits J, Hu L-F, Minarovits-Kormuta S, Klein G, Ernberg I. Sequence-specific methylation inhibits the activity of the Epstein-Barr virus LMP1 and BCR2 enhancer-promoter regions. Virology. 1994;200:661–667. doi: 10.1006/viro.1994.1229. [DOI] [PubMed] [Google Scholar]
- 61.Minarovits J, Minarovits-Kormuta S, Ehlin-Henriksson B, Falk K, Klein G, Ernberg I. Host cell phenotype-dependent methylation patterns of Epstein-Barr virus DNA. J Gen Virol. 1991;72:1591–1599. doi: 10.1099/0022-1317-72-7-1591. [DOI] [PubMed] [Google Scholar]
- 62.Mueller P R, Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989;246:780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- 63.Myöhänen S, Wahlfors J, Jänne J. Automated flourescent genomic sequencing as applied to the methylation analysis of the human ornithine decarboxylase gene. DNA Sequence. 1994;5:1–8. doi: 10.3109/10425179409039698. [DOI] [PubMed] [Google Scholar]
- 64.Niller H H, Hennighausen L. Formation of several specific nucleoprotein complexes on the human cytomegalovirus immediate early enhancer. Nucleic Acids Res. 1991;19:3715–3721. doi: 10.1093/nar/19.13.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niller H H, Glaser G, Knüchel R, Wolf H. Nucleoprotein complexes and DNA 5′-ends at oriP of Epstein-Barr virus. J Biol Chem. 1995;270:12864–12868. doi: 10.1074/jbc.270.21.12864. [DOI] [PubMed] [Google Scholar]
- 66.Nonkwelo C, Ruf I K, Sample J. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nonkwelo C, Ruf I K, Sample J. The Epstein-Barr virus EBNA-1 promoter Qp requires an initiator-like element. J Virol. 1997;71:354–361. doi: 10.1128/jvi.71.1.354-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996;70:623–627. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 70.Paulson E J, Speck S H. Differential methylation of Epstein-Barr virus latency promoters facilitates viral persistence in healthy seropositive individuals. J Virol. 1999;73:9959–9968. doi: 10.1128/jvi.73.12.9959-9968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puglielli M T, Woisetschlaeger M, Speck S H. oriP is essential for EBNA gene promoter activity in Epstein-Barr virus immortalized lymphoblastoid cell lines. J Virol. 1996;70:5758–5768. doi: 10.1128/jvi.70.9.5758-5768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rickinson A B, Young L S, Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987;61:1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robertson K D, Manns A, Swinnen L J, Zong J C, Gulley M L, Ambinder R F. CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt's lymphoma and Hodgkin's disease. Blood. 1996;88:3129–3136. [PubMed] [Google Scholar]
- 74.Robertson K D, Ambinder R F. Mapping promoter regions that are hypersensitive to methylation-mediated inhibition of transcription: application of the methylation cassette assay to the Epstein-Barr virus major latency promoter. J Virol. 1997;71:6445–6454. doi: 10.1128/jvi.71.9.6445-6454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robertson K D, Hayward S D, Ling P D, Samid D, Ambinder R F. Transcriptional activation of the Epstein-Barr virus latency C promoter after 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol. 1995;15:6150–6159. doi: 10.1128/mcb.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rooney C M, Brimmell M, Buschle M, Allan G, Farrell P J, Kolman J L. Host cell and EBNA-2 regulation of Epstein-Barr virus latent-cycle promoter activity in B lymphocytes. J Virol. 1992;66:496–504. doi: 10.1128/jvi.66.1.496-504.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowe D T, Rowe M, Evan G I, Wallace L E, Farrell P J, Rickinson A B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986;5:2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rowe M, Lear A L, Croom-Carter D, Davies A H, Rickinson A B. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66:122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruf I K, Sample J. Repression of Epstein-Barr virus EBNA-1 gene transcription by pRb during restricted latency. J Virol. 1999;73:7943–7951. doi: 10.1128/jvi.73.10.7943-7951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salamon D, Takacs M, Myöhänen S, Marcsek Z, Berencsi G, Minarovits J. De novo DNA methylation at nonrandom founder sites 5′ from an unmethylated minimal origin of DNA replication in latent Epstein-Barr virus genomes. Biol Chem. 2000;381:95–105. doi: 10.1515/BC.2000.014. [DOI] [PubMed] [Google Scholar]
- 81.Sample J, Henson E B D, Sample C. The Epstein-Barr virus nuclear protein 1 promoter active in type 1 latency is autoregulated. J Virol. 1992;66:4654–4661. doi: 10.1128/jvi.66.8.4654-4661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sample J, Brooks L, Sample C, Young L, Rowe M, Gregory C, Rickinson A, Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci USA. 1991;88:6343–6347. doi: 10.1073/pnas.88.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schaefer B C, Paulson E, Strominger J L, Speck S H. Constitutive activation of Epstein-Barr virus (EBV) nuclear antigen 1 gene transcription by IRF1 and IRF2 during restricted EBV latency. Mol Cell Biol. 1997;17:873–886. doi: 10.1128/mcb.17.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaefer B C, Strominger J L, Speck S H. Host-cell-determined methylation of specific Epstein-Barr virus promoters regulates the choice between distinct viral latency programs. Mol Cell Biol. 1997;17:364–377. doi: 10.1128/mcb.17.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sjöblom A, Jansson A, Yang W, Lain S, Nilsson T, Rymo L. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. J Gen Virol. 1995;76:2679–2692. doi: 10.1099/0022-1317-76-11-2679. [DOI] [PubMed] [Google Scholar]
- 86.Sjöblom A, Nerstedt A, Jansson A, Rymo L. Domains of the Epstein-Barr virus nuclear antigen 2 (EBNA2) involved in the transactivation of the latent membrane protein 1 and the EBNA Cp promoters. J Gen Virol. 1995;76:2669–2678. doi: 10.1099/0022-1317-76-11-2669. [DOI] [PubMed] [Google Scholar]
- 87.Sjöblom A, Yang W, Palmqvist L, Jansson A, Rymo L. An ATF/CRE element mediates both EBNA2-dependent and EBNA2-independent activation of the Epstein-Barr virus LMP1 gene promoter. J Virol. 1998;72:1365–1376. doi: 10.1128/jvi.72.2.1365-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sjöblom-Hallén A, Yang W, Jansson A, Rymo L. Silencing of the Epstein-Barr virus latent membrane protein 1 gene by the Max-Mad1-mSin3A modulator of chromatin structure. J Virol. 1999;73:2983–2993. doi: 10.1128/jvi.73.4.2983-2993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sung N S, Wilson J, Davenport M, Sista N D, Pagano J S. Reciprocal regulation of the Epstein-Barr virus BamHI-F promoter by EBNA-1 and an E2F transcription factor. Mol Cell Biol. 1994;14:7144–7152. doi: 10.1128/mcb.14.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sung N S, Kenney S, Gutsch D, Pagano J S. EBNA-2 transactivates a lymphoid-specific enhancer in the BamHI C promoter of Epstein-Barr virus. J Virol. 1991;65:2164–2169. doi: 10.1128/jvi.65.5.2164-2169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takacs M, Myöhänen S, Altiok E, Minarovits J. Analysis of methylation patterns in the regulatory region of the latent Epstein-Barr virus promoter BCR2 by automated fluorescent genomic sequencing. Biol Chem. 1998;379:417–422. doi: 10.1515/bchm.1998.379.4-5.417. [DOI] [PubMed] [Google Scholar]
- 91a.Takacs, M., D. Salamon, S. Myöhänen, H. Li, J. Segesdi, D. Ujvari, J. Uhlig, H. H. Niller, H. Wolf, G. Berencsi, and J. Minarovits. Epigenetics of latent Epstein-Barr virus genomes: high resolution methylation analysis of the bidirectional promoter region of latent membrane protein 1 and latent membrane protein 2B genes. Biol. Chem., in press. [DOI] [PubMed]
- 92.Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T. LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol. 1998;18:644–654. doi: 10.1128/mcb.18.1.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tao Q, Robertson K D, Manns A, Hildesheim A, Ambinder R F. The Epstein-Barr virus major latent promoter Qp is constitutively active, hypomethylated, and methylation sensitive. J Virol. 1998;72:7075–7083. doi: 10.1128/jvi.72.9.7075-7083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tao Q, Swinnen L J, Yang J, Srivastava G, Robertson K D, Ambinder R F. Methylation status of the Epstein-Barr virus major latent promoter C in iatrogenic B cell lymphoproliferative disease. Am J Pathol. 1999;155:619–625. doi: 10.1016/S0002-9440(10)65157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsang S F, Wang F, Izumi K M, Kieff E. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J Virol. 1991;65:6765–6771. doi: 10.1128/jvi.65.12.6765-6771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walls D, Perricaudet M. Novel downstream elements upregulate transcription initiated from an Epstein-Barr virus latent promoter. EMBO J. 1991;10:143–151. doi: 10.1002/j.1460-2075.1991.tb07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Waltzer L, Logeat F, Brou C, Israel A, Sergeant A, Manet E. The human Jκ recombination signal sequence binding protein (RBP-Jκ) targets the Epstein-Barr virus EBNA2 protein to its DNA responsive elements. EMBO J. 1994;13:5633–5638. doi: 10.1002/j.1460-2075.1994.tb06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang F, Gregory C, Rowe M, Rickinson A B, Wang D, Birkenbach M, Kikutani H, Kishimoto T, Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci USA. 1987;84:3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang F, Kikutani H, Tsang S F, Kishimoto T, Kieff E. Epstein-Barr virus nuclear protein 2 transactivates a cis-acting CD23 DNA element. J Virol. 1991;65:4101–4106. doi: 10.1128/jvi.65.8.4101-4106.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang F, Tsang S-F, Kurilla M G, Cohen J I, Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990;64:3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woisetschlaeger M, Jin W, Yandava C N, Furmanski L A, Strominger J L, Speck S H. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci USA. 1991;88:3942–3946. doi: 10.1073/pnas.88.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yates J L, Guan N. Epstein-Barr virus derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J Virol. 1991;65:483–488. doi: 10.1128/jvi.65.1.483-488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoo L I, Mooney M, Puglielli M T, Speck S H. B-cell lines immortalized with an Epstein-Barr virus mutant lacking the Cp EBNA2 enhancer are biased toward utilization of the oriP-proximal EBNA gene promoter Wp1. J Virol. 1997;71:9134–9142. doi: 10.1128/jvi.71.12.9134-9142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zetterberg H, Stenglein M, Jansson A, Ricksten A, Rymo L. Relative levels of EBNA1 gene transcripts from the C/W, F and Q promoters in Epstein-Barr virus-transformed lymphoid cells in latent and lytic stages of infection. J Gen Virol. 1999;80:457–465. doi: 10.1099/0022-1317-80-2-457. [DOI] [PubMed] [Google Scholar]
- 106.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang L, Pagano J S. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III latency. Mol Cell Biol. 1999;19:3216–3223. doi: 10.1128/mcb.19.4.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao B, Sample C E. Epstein-Barr virus nuclear antigen 3C activates the latent membrane protein 1 promoter in the presence of Epstein-Barr virus nuclear antigen 2 through sequences encompassing an Spi-1/Spi-B binding site. J Virol. 2000;74:5151–5160. doi: 10.1128/jvi.74.11.5151-5160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou S, Fujimuro M, Hsieh J J-D, Chen L, Hayward S D. A role for SKIP in EBNA2 activation of CBF1-repressed promoters. J Virol. 2000;74:1939–1947. doi: 10.1128/jvi.74.4.1939-1947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zimber-Strobl U, Kremmer E, Grasser F, Marschall G, Laux G, Bornkamm G W. The Epstein-Barr virus nuclear antigen 2 interacts with an EBNA2 responsive cis-element of the terminal protein 1 gene promoter. EMBO J. 1993;12:167–175. doi: 10.1002/j.1460-2075.1993.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zimber-Strobl U, Suentzenich K O, Laux G, Eick D, Cordier M, Calender A, Billaud M, Lenoir G M, Bornkamm G W. Epstein-Barr virus nuclear antigen 2 activates transcription of the terminal protein gene. J Virol. 1991;65:415–423. doi: 10.1128/jvi.65.1.415-423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zwicker J, Liu N, Engeland K, Lucibello F C, Müller R. Cell cycle regulation of E2F site occupation in vivo. Science. 1996;271:1595–1597. doi: 10.1126/science.271.5255.1595. [DOI] [PubMed] [Google Scholar]